Abstract
The optimum composition is determined for a nutrient medium corresponding to the maximum yield of bioethanol during the alcohol fermentation of the enzymatic hydrolyzate of lignocellulosic material, produced by treating oat hulls with a diluted solution of nitric acid in trial production. The biotechnological steps of saccharification and fermentation are performed using commercially available enzymatic preparations CelloLux-A and BrewZyme BGX, plus a strain of Saccharomyces сerevisiae Y-1693 (VKPM) that is resistant to inhibitors from hydrolyzates. The composition of a nutrient medium that produced a bioethanol yield which was 89.9% of the theoretical one (8.4% higher than the native hydrolyzate) is found to have a 1.82 g/L concentration of ammonium sulfate, a 0.98 g/L concentration of potassium monophosphate, and a 6.47 g/L concentration of yeast extract.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Since the discovery of cellulose’s chemical structure, the problem of producing monosugars from lignocellulosic biomass has been not only of scientific interest but important from both economic and social points of view. In the 20th century, the comprehensive use of freely available and annually renewable photosynthesized lignocellulosic biomass (biorefining) became one of the leading lines of biotechnology development, due to the successful introduction of the industrial conversion of monosugars into a wide range of compounds required for the world economy [1–3].
One of the most discussed products of biorefining nowadays is bioethanol; in addition, lignocellulosic raw materials have begun to replace sugar- and starch-containing raw materials for the production of technical alcohol. Transnational corporations produce bioethanol from sugarcane bagasse, cellulosic waste from the production of palm oil, the cellulosic components of fruits [4], straw [5], and cereal hulls [6]. Much progress has been made in the production of enzyme preparations (EP). The price of cellulases has dropped by a factor of 20 over the last 10 years [7, 8], so enzymatic transformation is currently the favored process. It is known that native lignocellulosic raw material is resistant to enzymatic hydrolysis and therefore must subjected to chemical, physical, or physicochemical treatment to activate it. Numerous authors have suggested multiple solutions, but the choice must be based not only on technology but on economy and ecology as well [9–11]. A number of compounds (furfural, oxymethylfurfural, ligno-humic substances, volatile organic acids, and others) can form during the activation of lignocellulosic material, which inhibits cellulases and the yeast zymase complex responsible for the conversion of glucose into ethanol. To alleviate this problem, steps of detoxification [7, 10] must be taken, or enzymes [7] and strains of microorganisms [12] that are insensitive to inhibitors must be used.
The technology for producing bioethanol from oat hulls, which includes pretreating the raw material with a diluted nitric acid solution, enzymatic hydrolysis of the resulting substrate in combination with alcoholic fermentation, and rectification of the produced bioethanol [13], was tested in a trial run at the Institute for Problems of Chemical and Energy Technologies, Siberian Branch, Russian Academy of Sciences. The uniqueness of nitric acid treatment is based on the reactions of hydrolysis, nitration, and oxidation occurring simultaneously, which results not only in the activation of the raw material to prepare it for the enzymatic hydrolysis of cellulose and hemicelluloses, but in lignin nitration as well. In addition, the presence water- and acid-insoluble lignin in the resulting substrate in the amount of 12.5 ± 0.1% does not reduce the yield of reducing compounds [14]. The use of commercially available enzyme preparations CelloLux-A and BrewZyme BGX, and of the non–genetially modified strain of yeast VKPM Saccharomyces сerevisiae Y-1693, made an additional contribution to the successful industrialization of the developed technology; the yield of bioethanol was 17.9 dcL/t, which was comparable to the current industrial productivity.
The possibilities for increasing the yield of bioethanol at every technological step must be explored in order to introduce this new technology to industry. Optimizing the nutrient medium is one such possibility at the stage of alcoholic fermentation. Even though this technological solution is commonly recognized, optimization must be performed for a particular nutrient medium and a particular strain-producer; variations depend on the features of the raw material, the chemical and enzymatic technologies for treating the raw material, and the strain-producer used in the specific biotechnological process [15, 16].
It is known that the hydrolyzates of lignocellulosic materials are generally not suitable for yeast cultivation, since they have insufficient amounts of nitrogen- and phosphorous-containing compounds, and they lack vitamins and growth stimulators [17]. In tests, we showed that the hydrolyzate of lignocellulosic material from oat hulls [13] used in this work does not meet industrial requirements in terms of the total number of cells and the fraction of budding cells [18], testifying to the deficit of nitrogen and vitamins in its composition.
The aim of this work was therefore to increase the yield of bioethanol by optimizing the composition of the nutrient medium produced via the enzymatic hydrolysis of lignocellulosic raw material from oat hulls (referred to below as hydrolyzate).
EXPERIMENTAL
Oat hulls were provided by ZAO Biiskii Elevator. Lignocellulosic material was produced in a trial run the Institute for Problems of Chemical and Energy Technologies, Siberian Branch, Russian Academy of Sciences, in a 250 L reactor at atmospheric pressure by treating oat hulls with a 4% solution of nitric acid [14]. The enzymatic hydrolysis of lignocellulosic material was the conducted in a 11 L fermenter with a substrate concentration of 60 g/L. Commercially available enzyme preparations (EPs) were used in two doses: 0.04 kg EP/kg of the substrate EP CelloLux-A, and 0.02 L EP/kg of the substrate EP BrewZyme BGX. EPs CelloLux-A (OOO PO Sibbiofarm, Berdsk, Russia) and BrewZyme BGX (Rusferment, Moscow, Russia) were standardized in cellulose and xylanase activities according to their manufacturers’ certificates. Enzymatic hydrolysis was performed at 46 ± 2°C for 42 h; active acidity was maintained at a level of 4.9 ± 0.2 pH units by manually adding orthophosphoric acid and ammonium hydroxide solutions. Hydrolyzate was separated from the solid residue upon completion of hydrolysis using vacuum filtration. The hydrolyzate was a transparent orange-yellow liquid of medium intensity; the concentration of reducing compounds in the hydrolyzate was 46.5 g/L, including 4.8 g/L of pentoses. The concentration of fermentable sugars was therefore 41.7%.
The concentration of reducing compounds in terms of glucose in the hydrolyzate was determined via spectrophotometry on a UNICO UV-2804 spectrometer (United States), using a reagent based on 3,5-dinitosalicilic acid (Panreac, Spain). The relative error of the technique was 3.45%. The concentration of pentoses in terms of xylose was determined spectrophotometrically according to a modified technique using an orcinol-ferric reagent [20].
We investigated the effect of three factors on the yield of bioethanol: the concentrations of ammonium sulfate, potassium monophosphate, and yeast extract (Table 1). Our selection of the factors was based on the need to add nitrogen- (in the form of ammonium sulfate) and phosphorous-containing (in the form of potassium monophosphate) nutrients, and on the familiar stimulation of yeast zymase complex by vitamins and growth factors, the cheapest source of which was yeast extract [17, 18]. Since our plan for a complete tree-factor experiment did not allow us to produce an adequate experimental statistical model, we performed additional experiments (see Table 1, lines 9–15). In addition to the indicated components, magnesium sulfate (1 g/L) and calcium chloride (0.2 g/L) were introduced into the media; their concentrations were the same in all experiments.
The prepared variants of nutrient media were sterilized in an autoclave (0.5 atm, 20 min) and inoculated with yeast following a sterility test. The VKPM strain of Saccharomyces сerevisiae Y-1693, cultivated for 24 h at 28°С in a sterile malt wort medium, was used as the inoculate. This strain was selected because of its unique resistance to hydrolyzates, the products of its own metabolism, and adverse conditions of cultivation [19], is the last being important for industrial applications. The doses of inoculate for different variants ranged from 8 to 12% in order to produce an initial total number of yeast cells in the nutrient medium of 11.0 million CFU/mL. The total number of yeast cells in the inoculate varied from 105 million to 135 million CFU/mL with 15 to 22% fractions of budding cells. The total number of yeast cells was determined using a Goryaev chamber. Fermentation was done under anaerobic conditions at 28°С.
The volumetric fraction of alcohol in the mush was determined areometrically in a distillate produced by distilling the mush according to GOST (State Satn-dard) 3639–79 [21]. The yield of alcohol was calculated from the concentration of fermented sugars, using the stoichiometric fermentation equation.
Each experiment was conducted in triplicate to produce satisfactory statistical data. The method of least squares was used to construct an experimental statistical model.
RESULTS AND DISCUSSION
The results from our experiments on optimizing the composition of hydrolyzate are presented in Table 1. It is known that the yield of ethanol in hydrolyzate plants is 55–58 L per 100 kg of fermented sugars [17], or 84.9–89.5% of the theoretical yield. In our experiments, the yield of bioethanol varied from 59.3% (the least successful experiments) to 88.9% (the maximum yield). The wide scatter of the values—29.6%—testifies to the strong dependence of the ethanol yield on the minerals and vitamins in the composition of the medium and the importance of the optimizing the composition.
The nonlinear pattern of the response was established as a result of complete three-factor experiment 23 (variants 1–8). Additional experiments (variants 9–15) were conducted to refine the type of the sought dependence. It was established by processing the experimental data that the dependence of mush strength Y on the composition of the medium was described by the equation
where Y is the strength of the mush, vol %; and X1, X2, X3 are dimensionless concentrations of ammonium sulfate, potassium monophosphate, and yeast extract, respectively.
The lower boundaries of the dimensionless factors (–1) correspond to the minimal concentrations of the nutrient medium’s components, i.e., 0 g/L for all three factors. The upper boundaries of the dimensionless factors (+1) correspond to the maximum concentrations of the components of the nutrient medium in the investigated range of values: 4 g/L for ammonium sulfate, 4 g/L for potassium monophosphate, and 20 g/L for yeast extract. The adequacy of the equation presented above was confirmed using the Fisher criterion [22] with a 0.05 level of significance. The confidence interval for Y was ±0.05.
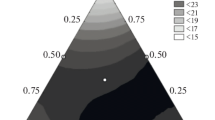
A graphic representation of the function of Y response is shown in Figure 1 as a set of cross sections of the hypercube. The change in mash strength depending on the composition of the nutrient medium is shown as the gradient of color intensity: the more intense color corresponds to stronger mash.
Analysis of resulting equation showed that the concentrations of ammonium sulfate and yeast extract were the greatest factors affecting the strength of the mush. The concentration of potassium phosphate in the range of 0 to 2 g/L did not affect the mash characteristics appreciably; in the range of 2 to 4 g/L, it reduced the yield and concentration of the mash. This is explained by the medium’s acidity during enzymatic hydrolysis being maintained with orthophosphoric acid, so the hydrolyzate contained sufficient amounts of phosphorous for the yeast’s nutrition. When hydrolysis is conducted in a phosphate buffer, we need not add this component to the nutrient medium, since the yield of bioethanol remains virtually the same even if it is not used.
The yield of bioethanol for the native hydrolyzate (variant 7) was 81.5%, testifying to the absence of inhibitors and technologically unfavorable compounds in the medium. Adjusting the vitamin–mineral composition allowed us to produce a quality nutrient medium like variant 11, where the yield was 88.9%, meeting industrial requirements.
We selected the optimum composition of the nutrient medium using a reduced gradient. The solution to the optimization problem showed that certain concentrations of the factors must be used in order to achieve the maximum yield and mash strength: ammonium sulfate, 1.82 g/L; potassium phosphate, 0.98 g/L; yeast extract, 6.45 g/L. These concentrations ensure a mash strength of 2.43 vol %, which corresponds to a bioethanol yield 89.9% of the theoretical one. Since (as was noted above) the concentration of potassium monophosphate has virtually no effect on the mash’s characteristics (so long as enzymatic hydrolysis is conducted in a phosphate buffer), excluding it allows us to produce mash with a yield of 89.6% and strength of 2.42 vol %.
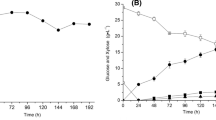
To evaluate the efficiency of alcoholic fermentation depending on the medium’s composition, we calculated the rate constants of substrate consumption presented in Table 1. All of the constants are very close (from 49.5 × 10−3 in variant 1 to 50.6 × 10−3 in variant no. 11), testifying to the similar biochemical activity of the yeast in all variants. The residual concentration of reducing compounds in the medium (the concentrations of nonfermentable pentoses (4.8 g/L) and residual glucose concentration) can serve as an indirect indicator of the efficiency of fermentation [23]. The residual concentration of reducing compounds in our experiments varies from 11.1 g/L (in variant 1) to 8.4 g/L (in variant 11). These are relatively close values. In addition, noted that the higher the reaction constant, the lower the residual concentration of reducing compounds. The data on the change in the number of yeast cells during fermentation are not presented, since there was no correlation between the number of yeast cells, the fraction of budding cells, and the yield of ethanol. Analysis of the concentration of alcohol in the mash gives the best picture of the efficiency of alcoholic fermentation, and the increase in the yield of bioethanol testifies to the coordinated work of the yeast zymase complex in the selected optimum composition of the nutrient medium.
CONCLUSIONS
The dependence of the yield of bioethanol on the composition of a nutrient medium based on the enzymatic hydrolyzate of lignocellulosic material produced in trial runs by treating oat hulls with a diluted nitric acid solution under atmospheric pressure was demonstrated. A regression equation was constructed in order to clarify the interrelation between concentrations of ammonium sulfate, potassium monophosphate, and yeast extract. Correlation coefficients for the equation were calculated using the method of least squares. It was established that the equation adequately described the experimental data with a significance level of 0.05.
The composition of the nutrient medium was determined by solving the problem of optimization, which ensured the maximum yield and mash strength: concentration of ammonium sulfate, 1.82 g/L; of potassium monophosphate, 0.98 g/L; of yeast extract, 6.47 g/L. Under the above conditions, the strength of the mush was 2.43 vol %, which corresponded to a bioethanol yield that was 89.9% of the theoretical one.
The resulting composition of additives to the nutrient medium allowed the yield of bioethanol to be increased by 8.4%, compared to using native hydrolyzate, and is recommended for industrial use.
REFERENCES
Biorefineries—Industrial Processes and Products/ Status Quo and Future Directions, Kamm, B., Gruber, P.R., and Kamm, M., Eds., Weinheim: WILEY-VCH, 2010.
Mussatto, S.I., Dragone, G., Guimarães, P.M.R., Silva, J.P.A., Carneiro, L.M., Roberto, I.C., Vicente, A., Domingues, L., and Teixeira, J.A., Biotechnol. Adv., 2010, vol. 28, no. 6, pp. 817–830.
Balat, M., Balat, H., and Öz, C., Prog. Energy Combust. Sci., 2008, vol. 34, no. 5, pp. 551–573.
Global solutions/Inbicon. http://www.inbicon.com/en. Cited July 16, 2018.
Larsen, J., Østergaard Petersen, M., Thirup, L., Wen Li, H., and Krogh Iversen, F., Chem. Eng. Technol., 2008, vol. 31, no. 5, pp. 765–772.
Lawford, H.G., Rousseau, J.D., and Tolan, J.S., Appl. Biochem. Biotechnol., 2001, vol. 91, nos. 1–9, pp. 133–146.
Agrawal, R., Satlewal, A., Gaur, R., Mathur, A., Kumar, R., Gupta, R.P., and Tuli, D.K., Biochem. Eng. J., 2015, vol. 102, pp. 54–61.
McMillan, J.D., Jennings, E.W., Mohagheghi, A., and Zuccarello, M., Biotechnol. Biofuels, 2011, vol. 4, pp. 29–46.
Brodeur, G., Yau, E., Badal, K., Collier, J., Ramachandran, K.B., and Ramakrishnan, S., Enzyme Res., 2011, vol. 2011. http://dx.doi.org/10.4061/2011/787532
Hu, F. and Ragauskas, A., BioEnergy Res., 2012, vol. 5, no. 4, pp. 1043–1066.
Podgorbunskikh, E.M., Bychkov, A.L., and Lomovskii, O.I., Catal. Ind., 2016, vol. 8, no. 3, pp. 274–279.
Saha, B.C., Nichols, N.N., Qureshi, N., Kennedy, G.J., Iten, L.B., and Cotta, M.A., Bioresour. Technol., 2015, vol. 175, pp. 17–22.
Baibakova, O.V., Skiba, E.A., Budaeva, V.V., and Sakovich, G.V., Catal. Ind., 2017, vol. 9, no. 3, pp. 257–263.
Skiba, E.A., Budaeva, V.V., Baibakova, O.V., Zolotukhin, V.N., and Sakovich, G.V., Biochem. Eng. J., 2017, vol. 126, pp. 118–125. http://dx.doi.org/ doi 10.1016/j.bej.2016.09.003
Gracheva, I.M. and Krivova, A.Yu., Tekhnologiya fermentnykh preparatov (Technology of Enzyme Preparations), Moscow: Elevar, 2000.
Huang, Y., Qin., X., Luo, X.-M., Nong, Q., Yang, Q., Zhang, Z., Gao., Y., Lv, F., Chen, Y.,Yu., Z., Liu, J.-L., and Feng, J.-X., Biomass Bioenergy, 2015, vol. 77, pp. 53–63.
Khol'kin, Yu.I., Tekhnologiya gidroliznykh proizvodstv. Uchebnik dlya vuzov (Technology of Hydrolytic Production: Textbook for Universities), Moscow: Lesnaya promyshlennost’, 1989.
Rimareva, L.V. and Vorontsova, N.N., Mikrobiologi-cheskii kontrol’ spirtovogo i fermentnogo proizvodstv (Microbial Control for Alcohol and Enzyme Production), Moscow: Rossel’khozakademiya, 2005.
Skiba, E.A. and Baibakova, O.V., Polzunovskii Vestn., 2013, no. 3, pp. 214–219.
GOST (State Standard) 10820-75: Pulp. Method for Determination of Pentosans Fraction of Total Mass, Moscow: Izd. Standartov, 1991.
GOST (State Standard) 3639-79: Water-Alcohol Solutions. Methods for the Determination of the Ethyl Alcohol Content, Moscow: Izd. Standartov, 2004.
Gmurman, V.E., Teoriya veroyatnostei i matematicheskaya statistika: Uchebnoe posobie dlya vuzov (Theory of Probability and Mathematical Statistics: Textbook for Universities), Moscow: Vysshaya Shkola, 2003.
Yarovenko, V.L., Marinchenko, V.A., Smirnov, V.A., Ustinnikov, B.A., Tsygankov, P.S., Shvets, V.N., and Belov, N.I., Tekhnologiya spirta (Alcohol Technology), Yarovenko, V.L., Ed., Moscow: Kolos, 2002.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Brovko
Rights and permissions
About this article
Cite this article
Skiba, E.A., Mironova, G.F., Kukhlenko, A.A. et al. Enhancing the Yield of Bioethanol from the Lignocellulose of Oat Hulls by Optimizing the Composition of the Nutrient Medium. Catal. Ind. 10, 257–262 (2018). https://doi.org/10.1134/S207005041803008X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207005041803008X