Abstract
A study of the frequency of deformations of chironomid larvae and correlation with water quality in the Chi River basin, Maha Sarakam Province, Thailand was carried out. The chironomid larvae and water samples were collected in six sampling sites. Four sampling sites were located in rural areas and the other two sites were located in urban areas. The results showed that the mean values of dissolved oxygen (DO) and biochemical oxygen demand (BOD5) were significantly different among sampling sites (p < 0.05). Whereas, the mean values of water temperature, pH, electrical conductivity, and total dissolved solid were not significantly different among sampling sites. A total of 148 individuals of larvae were sampled comprising 8 genera belonging to 3 subfamilies; Chironominae (75.7%, 5 genera), Orthocladiinae (14.2%, one genus), and Tanypodidae (10.1%, 2 genera). The result indicated that the majority of genera; Kiefferulus sp., Chironomus sp., and Polypedilum sp. were discovered. Genus Polypedilum was obtained in the highest number of subfamily Chironominae. This study showed that the incidence of larvae deformity strongly correlated with DO level. Site MK3 (pond at engineering building) which had the highest DO level showed the significantly highest incidence of chironomid deformity at 36.4%, followed by sites MK5 (Kaeng Loeng Chan reservoir site 1) (12.9%), MK1 (Nong Bua reservoir) (11.1%), MK6 (Kaeng Loeng Chan reservoir site 2) (11.1%), and MK4 (pond at science building) (10.9%). In conclusion, deformities of chironomid larvae could be used indirectly as a potential biomonitoring tool for detecting water quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The macroinvertebrates that spend most of their lives in water are considered as ideal organisms for biomonitoring. They are sometimes exposed to many toxicants, including heavy metals, inorganic substances, and organic pesticides. Chironomids (Insecta: Diptera: Chironomidae) are the most abundant freshwater invertebrates especially on sediment surfaces. They are known as non-biting midges and they are known to have a specific tolerance. Because of this, they are well suitable to monitoring polluted aquatic systems. (Warwick, 1990; Armitage et al., 1995; Cranston, 2004).
Abnormalities in larval chironomids occur in the region of mouthparts, mainly the mentum, and are also found in other structures such as epipharynx, and mandible. An analysis of deformities in the chironomid larvae has been performed since early 1970. Particularly, previous studies have shown deformities in chironomid larvae associated with contaminated sediments (Hamilton and Saether, 1971; Warwick, 1985, 1988, 1989; Lenat, 1993). However, the incidence of the mentum deformities represents a sub-lethal response to stream and rivers pollutants. Thus, these deformities are considered an early warning indicator of water quality deterioration (Odume et al., 2012). Causes of deformities in the larvae were investigated to gain a potential tool for pollution monitoring programs, especially for the detection of heavy metals from industrial pollutants (Janssens et al., 1992). In addition, other pollutants from agriculture and urban areas can cause deformed larvae (Bird, 1994). Lenat (1993) showed that the severity of mentum deformities could be used as a criterion to evaluate the effects of pollution and was scored using Lenat’s toxic score index (TSI).
In many reports, it was problematic to indicate causative agents for the incidence of larvae deformities so a study of the incidence of chironomid deformities is essential to understand the relationship between pollutants and types of deformities. The effect of heavy metals and polychlorinated biphenyl (PCBs) on chironomid deformities was reviewed by many reports (Warwick, 1990; Janssens et al., 1992; Thomas et al., 1993; Alessandra et al., 2010; Alessandra et al., 2014; Francis et al., 2018). The Chi River basin, covers seven provinces of northeastern Thailand. It is the longest river in this region and is an essential freshwater ecosystem for agriculture, irrigation, aquaculture, and livestock. For a decade, the Chi River has encountered a quality problem with several contaminants, including agricultural runoff, pesticides, and municipal sewage. The incidence of chironomid deformities is a valuable tool for monitoring water ecosystems, as suggested by several reports (Wise et al., 2001; Ochieng et al., 2008; Odume et al., 2012 and Akyildiz et al., 2018). Recently, some pioneer studies on biology, taxonomy, ecology and cytogenetics of chironomids, including the incidence of chironomid deformities, have been conducted in several streams and rivers in Thailand (Hashimoto et al., 1981; Mustow et al., 2002; Cranston, 2007; Utayopas, 2011; Simwisat et al., 2015; Thani and Prommi, 2017; Weeraprapan et al., 2018). In the Chi River basin, biological assessment has been carried out on the diversity of chironomid larvae to assess water quality in the Phong River (Sriariyanuwath et al., 2015). However, no reports of chironomid deformities were presented.
This research is the first report of chironomid deformities in the Chi River basin in Thailand. The present study aimed to show the relationship between water quality and the incidence of deformity of the mentum of chironomids.
MATERIALS AND METHODS
Study area and sampling sites. The Chi River originates from Petchaboon hills, which are located at 16°40′36.2″ N latitude and 102°2′29″ E longitude in the northeastern of Thailand (Fig. 1) and its basin is approximately 49 480 km2. The river runs east through six provinces; Chaiyaphum, Khon Kaen, and Maha Sarakham, then turns the south in Roi-Et, runs through Yasothon, and joins the Mun River in the Kanthararom district, Sisaket Province. The Chi River receives water from many small rivers that receive urban, agricultural and domestic wastes that it discharged directly into the river. There are several waste substances involved, including pesticides, organic and inorganic wastes from the livestock.
Four and two water bodies from reservoirs and oxbow lakes, respectively in Maha Sarakham province were selected to collect chironomid larvae and water quality assessment (Fig. 1). The location of each sampling site was in areas with different surrounding land-use activities (rural or urban). There were four large reservoirs, MK1 (Nong Bua1), MK2 (Nong Bua2), MK5 (Kaeng Loeng Chan 1), and MK6 (Kaeng Loeng Chan 2), which were located in a rural area and contained many water plants. Site MK1 and MK2 received some discharges from nearby communities of Kantarawichai district. These sites also received agricultural discharge and wastewater from residential units nearby these areas. Site MK5 and MK6 were oxbow lakes contaminated with organic wastes from agricultural and anthropogenic activities.
Two small reservoirs, MK3 (pond at engineering building) and MK4 (pond at Science building) were located in Mahasarakham University (MSU), which is an urban area. These sites mainly received wastewater from human activity on the campus. On each reservoir, chironomid larvae and water samples were collected on three occasions from December 2019 to January 2021.
Physicochemical variables of water. On each sampling occasion, measurements of physicochemical parameters in the field; specifically water pH, water temperature, dissolved oxygen (DO), total dissolved solids (TDS), electrical conductivity (EC), and biochemical oxygen demand (BOD5) were conducted in situ at two randomly selected locations at each sampling site. The water temperature, TDS, and EC were measured with TDS/EC meter, and pH was measured with a pH meter. The dissolved oxygen was measured by titration method, and the water samples from each site were collected using 300-mL BOD bottles from the previously described method (APHA, 2017). Each parameter was measured in triplicate. All water samples were transported to the laboratory in an icebox and kept at 4°C until analyzed.
Chironomid larval sampling. Chironomid larvae were randomly collected by Ekman grab sampler and about 100 m transect in each site. In addition, a D‑framed aquatic net (40 cm wide, 300-µm mesh size) was used for collecting the larval samples. All benthic macroinvertebrates collected at each site were transferred to plastic bags containing 80% ethanol. The samples were washed with tap water through a sieve (300-µm pore). The residue on the sieve was transferred into a white plastic pan and tap water was added to the pan. Then, the macroinvertebrates and chironomid larvae were separated and kept in small vials. The larval samples were preserved in 80% ethanol for subsequent taxonomic identification.
Examination of chironomid deformities. Permanent slides of chironomid larvae were prepared to examine the morphological deformities of each larva. The preserved larvae were transferred to the petri dish containing 10% KOH solution and left in the solution for 24 h. After that, the larvae were prepared on permanent slides following the method of Epler (2001). The slide-mounted larvae were identified to genus level using standard taxonomic keys (Warwick, 1990; Epler, 2001; Cranston, 2004). Larval head capsules of each genus were examined for their deformities under a compound microscope at X400 magnification. The mentum was examined for the occurrence of deformities.
Data analysis. The physicochemical variables were compared using a one-way analysis of variance (ANOVA) (p < 0.05). All data were log(X + 1) transformed, and ANOVA was used to compare differences between sites. Data were analyzed using SPSS 20 for Windows. Community incidences of mentum deformities were calculated as the percentage of all deformed larvae to the total larvae for each site. Pearson bivariate correlation (P = 0.05) was used to demonstrate the relationship between chironomid deformities and physicochemical variables in water.
RESULTS
Physicochemical variables of water quality. Means, standard deviations, and ranges of physicochemical variables measured at the six sampling sites were obtained (Table 1). ANOVA conducted on log(X + 1) transformed data revealed that the mean values of DO and BOD were significantly different (p < 0.05) among sampling sites. The result showed that the mean values of water temperature, pH, EC, and TDS were not significantly different (p > 0.05) for all sites.
The mean temperature was 27.8°C, and it ranged between 22.5 and 33.2°C. The mean of DO increased in the lentic systems due to photosynthesis by the algal population. The DO was slightly low (mean 2.92 mg/L). The highest value of DO was found in site MK3, while the lowest value was found in MK2.
Naturally, the pH value in each reservoir differs according to riparian vegetation and the surrounding land use. In this study, the mean of the pH was slightly basic at most sites which range from 6.7 to 13.1. The pH values of MK1, MK4, and MK5 were considerably variable in each season with a high standard deviation. The pH value was influenced by the metabolic activities of the flora particularly photosynthesis and the presence of substantial amounts of organic matter from terrestrial sources, which also affect water pH. The pH range of MK2 and MK3 sites, which were small reservoirs and received water from municipals, was narrow with a low standard deviation.
The mean EC was 209.83 µS/cm. The lowest value was found in site MK6, while site MK3 and site MK4 showed relatively higher values. The TDS value of all sampling areas was consistent with the EC values, with a mean value of 118 ± 24.6 mg/L.
Deformity of Chironomid larvae. A total of 148 chironomid larvae from the six sampling sites during the three sampling occasions were screened for deformities in the mentum. Individuals of the tribe Chironomini were more deformed than members of other subfamilies and tribe Tanytarsini (Table 2). The community incidences of mentum deformity were highest at sites MK3 throughout the sampling seasons and were higher than 36%, indicating pollution sites in Mahasarakham University campus, followed by sites MK5, MK6, MK1 and, MK4 (13, 11, 11, and 11%, respectively). However, only two individual larvae with normal mentum were obtained from site MK2 (Table 2).
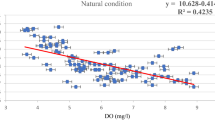
Correlation between deformity of mentum and water physicochemical variables. The effect of water quality variables on the incidence of deformation of chironomid larvae was analyzed using Pearson correlation. DO values influenced the deformities of the larvae in the site MK3 with a positive correlation (p < 0.05; r = 0.83) (Table 3). However, other variables did not affect the larval deformity that correlation coefficient showed weak relationships (r = –0.10–0.71) that were considered correlated importance of the value at least 0.8. Furthermore, the water temperature and the number of larvae were the main factors determining the incidence of deformation (Table 3).
Deformity types. The chironomid larvae in this study showed a variety of deformity types, including missing teeth, extra teeth, and lateral gaps. The missing teeth and extra teeth were the most types of deformities (Fig. 2). The highest incidence of deformity was found in site MK3 (36.4%), which was usually the threshold of water body contamination. Moreover, other sites showed relatively high (>10%) except in the MK2, which did not find larval deformity.
DISCUSSION
The physicochemical parameters of water quality are shown in Table 1. The DO and BOD5 values are essential to indicate levels of pollution. DO depletion could affect chironomid deformities (Nazarova et al., 2004). From this study, DO values influenced the deformities of the larvae while there was no relationship between the concentration levels of other variables and chironomid larva deformities. The values of the selected variables at all sampling sites, which were in the range of the standard surface waters quality (SSWQ) of Thailand might have minimal effect on chironomid larva deformities (Pollution Control Department, PCD 2000). Based on the SSWQ, all study sites were classified into water quality class 3 (fair quality) used for consumption by passing through an ordinary treatment process.
The significantly low DO in site MK2 with relatively high BOD5 level in MK3 showed significant differences compared with other sampling sites (p < 0.05). Site MK2 was a large reservoir that contained many water plants, fishes, birds, and ducks. In addition, many agricultural farms surrounded this area. This reservoir received various organic waste from animals and agricultural purposes. In addition, this reservoir was a natural catchment, which was lentic water. Although site MK1 was located in the same area of MK2, BOD5 of this site was much lower than site MK2. This might be due to site MK1 being far away from the agricultural area and containing fewer water plants. Site MK3 was located in the MSU campus, which wastewater contaminated some organic materials from human activity ran through the reservoirs. In this study, low DO levels showed a strong positive correlation with the incidence of larval deformity. However, it was reported that DO level was not a significant factor for the chironomid larvae deformity due to the hemoglobin pigment in their bodies (Cranston, 2004). Armitage et al. (1995) reported that some species of chironomid larvae could tolerate limited conditions in depleted oxygen environments.
Statistical results showed that the mean values of water temperature, pH, electrical conductivity, and TDS were not significantly different among sampling sites (p > 0.05). The mean pH of most sites was slightly basic, which the mean values were within the range (5.00–9.00) of the standard surface water quality of Thailand (PCD, 2000). The mean EC at sites MK3 and MK4 showed a relatively high value (300–309.5 µS/cm). These EC depended on the inflow water received from the surrounding area, which contained many ions contributing to the conductivity comprising sodium, potassium, calcium, and chloride. The TDS value was consistent with the EC value; it ranged between 73.7 and 139 mg/L. The highest value of TDS was measured in site MK 3, which also contained the highest BOD5 level. However, the mean value of TDS in this study was lower than the report of the Phong River in Thailand, which was contaminated from pulp factory waste (Sriariyanuwath, 2015).
The chironomid larvae of the six sampling sites mainly consisted of Chironominae (75.6% of total number of larvae, five genera) followed by Orthocladiinae (14.2%, one genus) and Tanypodinae (10.1%, two genera). Kiefferulus spp., Chironomus spp., and Polypedilum spp. were dominant genera and only Kiefferulus spp. was found at all sampling sites. The highest number of Polypedilum spp. was discovered in Chironominae. It has been reported that this genus was common in tropical waters, including Thailand (Cranston, 2007). In Thailand, 3 subfamilies and 48 genera of chironomid larvae have been reported (Mustow et al., 2002; Cranston, 2007). In the present study, subfamily Chironominae showed a higher incidence of deformities compared with taxa in the other subfamilies, Orthocladiinae and Tanypodinae (Table 2). However, incidences of deformities varied among genera of chironomid larvae in all study sites. The result showed that members of Tanypodinae were comparatively low numbers in most sampling sites. (Berg, 1995) stated that these taxa were mostly predators, and their feeding habits less contracted to the polluted sediment. Several studies have shown low incidences of deformities in family Tanypodinae (Odume et al., 2012; Weeraprapan, 2018). On the other hand, the tribe Chironomini showed relatively high incidence of deformity because these taxa were mostly detrital feeders (Berg, 1995; Cranston, 2004, 2007; Sriariyanuwath, 2015), and their life is cycle closely exposed to polluted sediment (Odume et al., 2012).
The chironomid taxa in most sites showed several deformity types. These included missing teeth, fused teeth, extra teeth, and lateral gaps. Most types of deformities in this study involved missing teeth and fused teeth. MacDonald and Taylor (2006) stated that it was not clear about the relationship between the type of deformities and particular contaminants. In this study, site MK3 with the highest BOD5 level showed the significantly the highest incident of chironomid deformity of 36%, followed by sites MK5, MK6, and MK4 (13, 11, 11, and 11%, respectively). However, at site MK2, only two individuals were found with normal mentum.
According to the studies of Grebenjuk and Tomilina (2014) and Tomilina and Grebenjuk (2019) who studied the deformities of the genus Chironomus spp. reported the negative impact of organic toxicants leading to mentum and mandible deformalities of larvae. The number of these deformities significantly increased in natural water bodies receiving long-term pollution by industrial and agricultural waste waters.
Correlation values 0.7 up to 0.9 assess as one (high) level of relation. The Pearson correlation between percentage of frequency of deformation and temperatures, DO value and number of larvae were 0.71, 0.83 and 0.71 respectively. Although, Chrinomid larvae could live in both good and poor water quality, this result reveals that there were less numbers of larvae in water with high BOD concentration (Pearson correlation = –0.46). Also, there were high number of Chrinomid larvae with high DO value (Pearson correlation = 0.37). In this study, the more larvae were found, the higher frequency of deformation was present.
Naturally, the incidences of deformities in chironomid larvae have been reported to be <1%, compared to the sites contaminated with metal in Canada with incidence >1% (Swansburg et al., 2002). In USA and Canada, reports showed that incidences of deformities in chironomid larvae reared in clean sediments were relatively low (0.9–2.2%), whereas those of larvae reared in sediments contaminated with heavy metals ranged from 3.8 to 10.3%. The findings of several reports revealed the correlation of deformed chironomid incidences in rural and anthropogenic contaminated areas, which contaminated with heavy metals and pesticides in the surrounding catchments activities (Warwick, 1990; Wise et al., 2001; Ochieng et al., 2008; Odume et al., 2012; Veroli et al., 2014; Arimoro et al., 2017; Youbi et al., 2020).
CONCLUSIONS
The chironomid larvae in the subfamily Chironominae were the highest numbers in all sampling sites. The most frequent types of larval deformities included missing teeth and fused teeth. This study showed the correlation of deformed chironomid incidence with poor water quality. Physicochemical variables measured in six sampling sites of the Chi River did not exceed the values of the standard of Thailand. DO and BOD5 level were correlated with EC and TDS. The Chi River catchments in Kantarawichai and Muang Districts, Maha Sarakham province, Thailand need to be monitored closely to minimize waste-inflow into the surrounding aquatic systems. Deformities of chironomid larvae were beneficial as a potential bio-indicator for water quality assessment.
REFERENCES
Akyildiz, G.K., Bakir, R., Polat, S., et al., Mentum deformities of chironomid larvae as an indicator of enviromental in büyük Menders river, Turkey, Inland Water Biol., 2018, vol. 11, pp. 515‒522. https://doi.org/10.1134/S199508291804002
Alessandra, D.V., Roberta, S., Roberto, M.P., et al., Sediment toxicity and deformities of chironomid larvae in Lake Piediluco (Central Italy), Chemosphere, 2010, vol. 79, no. 1, pp. 33–39.
Alessandra, D.V., Santoro, F., Pallottini, M., et al., Deformities of chironomid larvae and heavy metal pollution: From laboratory to field studies, Chemosphere, 2014, vol. 112, pp. 9–17.
APHA (America Public Health Association), American Water Works Association and Water Pollution Control Federation, Standard Methods for the Examination of Water and Wastewater, America Public Health Association, 2017.
Arimoro, F., Yohanna I.A., Odume, N., et al., Mouthpart deformities in Chironomidae (Diptera) as bioindicators of heavy metals pollution in Shiroro lake, Niger state, Nigeria, Ecotoxicol. Environ. Saf., 2017, vol. 149, pp. 96–100.
Armitage, P.D., Cranston, P.S., and Pinder, L.C.V., The Chironomidae: Biology and Ecology of Non-Bitting Midges, Armitage, P.D., Cranston, P.S., and Pinder, L.C.V., Eds., London: Chapman & Hall, 1995.
Berg, M.B., Larval food and feeding behavior, in The Chironomidae: Biology and Ecology of Non-Bitting Midges, Armitage, P.D., Cranston, P.S., and Pinder, L.C.V., Eds., London: Chapman & Hall, 1995, pp. 136‒168.
Bird, G.A., Use of chironmid deformities to assess environmental degradation in the Yamaska River, Quebec, Environ. Monit. Assess., 1994, vol. 30, pp. 163–175.
Cranston, P.S., Insecta: Diptera, Chironomidae, in The Freshwater Invertebrate of Malaysia and Singapore, Yule, C.M. and Yong, H.S., Eds., Malaysia: Academic of Sciences, 2004.
Cranston, P.S., The Chironomidae larvae associated with the tsunami-impacted waterbodies of the coastal plain of Southwestern Thailand, Raffles Bull. Zool., 2007, vol. 55, no. 2, pp. 231–244.
Cranston, P.S., Introduction in armitage, in The Chironomidae: Biology and Ecology of Non-Bitting Midges, Armitage, P.D., Cranston, P.S., and Pinder, L.C.V., Eds., London: Chapman & Hall, 1995.
Epler, J.H., Identification manual for the larval Chironomidae (Diptera) of North and South Carolina. A guide to the taxonomy of the midges of the southeastern United States, including Florida. Special Publication SJ2001-SP13. North Carolina Department of Environmental and National Resources, Releigh, NC, 2001.
Francis, O.A., Yohanna, I.A., Oghennekaro, N.O., et al., Mouthpart deformities on Chironomidae (Diptera) as bioindicators of heavy metals pollution in Shiroro Lake, Niger State, Nigeria, Ecotoxicol. Environ. Saf., 2018, vol. 149, pp. 96–100.
Grebenjuk, L. and Tomilina, I.I., Morphological deformations of hard-chitinized mouthpart structures in larvae of the genus Chironomus (Diptera, Chironomidae) as the index of organic pollution in freshwater ecosystems, Inland Water Biol., 2014, vol. 7, pp. 273–285. https://doi.org/10.1134/S1995082914030092
Hashimoto, H., Wognsiri, T., Wongsiri, N., et al., Chironomidae from rice fields of Thailand with description of 7 new species, Tech. Bull., Entomol. Zool. Div., Dep. Agric., Bangkok, Thailand, 1981, vol. 7, pp. 1–47.
Hamilton, A.L. and Saether, O.A., The occurrence of characteristics deformities in the chironomid larvae of several Canadian lakes, Can. Entomol., 1971, vol. 103, no. 3, pp. 363–368.
Janssens de Bisthosven, L.G., Timmermans, R., and Ollevier, F., The concentration of cadmium, lead, copper and zinc in Chironomus gr. thummi larvae (Diptera, Chrionomidae) with deformed versus normal menta, Hydrobiologia, 1992, vol. 239, pp. 141–149.
Lenat, D.R., Using mentum deformities of chironomus larvae to evaluate the effects of toxicity and organic loading in streams, J. North Am. Benthol. Soc., 1993, vol. 12, no. 3, pp. 265–269.
MacDonald, E.E. and Taylor, B.R., Incidence of mentum deformities in midge larvae (Diptera: Chironomidae) from Northern Nova Scotia, Canada, Hydrobiologia, 2006, vol. 563, pp. 277–287.
Mustow, S.E., Wilson, R.S., and Gotchagorn, S., Chironomid assemblages in two Thai watercourse in relation to water quality, Nat. Hist. Bull. Siam Soc., 2002, vol. 45, no. 53–64.
Nazarova, L., Riss, H., Kahlheber, A., et al., Some observations of buccal deformities in chironomid larvae (Diptera: Chironomidae) from the Ciénaga Grande de Santa Marta, Colombia, Caldasia, 2004, vol. 26, no. 1, pp. 275–290.
Ochieng, H., de Ruyter van Steveninck, E., and Wanda, F., Mouthpart deformities in Chironomidae (Diptera) as indicators of heavy metal pollution in northern Lake Victoria, Uganda, Afr. J. Aquat. Sci., 2008, vol. 33, no. 2, pp. 135–142.
Odume, N., Muller, W., Palmer, C., et al., Mentum deformities in Chironomidae communities as indicators of anthropogenic impacts in Swartkops River, Phys. Chem. Earth., 2012, vols. 50–52, pp. 140–148.
PCD (Pollution Control Department). Water quality standard and criteria in Thailand. Pollution Control Department, Ministry of Science Technology and Environment, Bangkok, Thailand, 2000.
Simwisat, K., Uttaruk, P., and Pramual, P., Morphology, cytogenetics and DNA barcode of the Chironomidae (Diptera) in Thailand, J. Sci. Technol. MSU, 2015, vol. 34, p. 74.
Sriariyanuwath, E.O, Sangpradub, N., and Hanjavanit, C., Diversity of chironomid larvae in relation to water quality in the Phong River, Thailand, AACL Bioflux, 2015, vol. 8, no. 6, pp. 933–945.
Swansburg, E.O., Wayne, L.F., Brian, J.F., and Jan, J.H., Mouthpart deformities and community composition of Chironomidae (Diptera) larvae downstream of metal mines in New Brunswick, Canada, Environ. Toxicol. Chem., 2002, vol. 21, no. 12, pp. 2675–2684.
Thani, I. and Prommi, T., Mentum deformities in Chironomidae (Diptera, Insecta) as indicator of environmental perturbation in freshwater habitats, Jordan J. Biol. Sci., 2017, vol. 10, pp. 229–233.
Thomas, P. and Kenton, M.S., Deformities of aquatic larval midges (Chironomidae: Diptera) in the sediments of the Buffalo River, New York, J. Great Lakes Res., 1993, vol. 19, no. 4, pp. 648–659.
Tomilina, I.I., Grebenjuk, L.P., Induction of deformities of the hard-chitinized mouthpart structures of larvae Chironomus riparius Meigen under various contents of persistent organic substances in bottom sediments, Inland Water Biol., 2019, vol. 12, no. 3. pp. 346–355. https://doi.org/10.1134/S1995082919030167
Utayopas, P., Diversity of Chironomidae larvae in Pratumthani Province, Thailand, J. Sci. Tech., 2011, vol. 19, p. 18.
Veroli, A., Santoro, F., Pallottini, M., et al., Deformities of chironomid larvae and heavy metal pollution: From laboratory to field studies, Chemosphere, 2014, vol. 112, pp. 9–17.
Warwick, W.F., Morphological abnormalities in Chironomidae (Diptera) larvae as measures of toxic stress in freshwater ecosystems: indexing antennal deformities in Chironomus Meigen, Can. J. Fish Aquat. Sci., 1985, vol. 42, pp. 1881–1914.
Warwick, W.F., Morphological deformities in Chironomidae (Diptera) larvae as biological indicators of toxic stress, in Toxic Contaminants and Ecosystem Health; A Great Lake Focus, Evan, M.S., Ed., New York: John Wiley and Sons, 1988.
Warwick, W.F., Morphological deformities in larvae of Procladius Skuse (Diptera: Chironomidae) and their biomonitoring potential, Can. J. Fish. Aquat. Sci., 1989, vol. 46, pp. 1255–1270.
Warwick, W.F., The use of morphological deformities in chironomid larvae (Diptera: Chironomidae) for biological effects monitoring, Environ. Can. Inl. Waters Direct. Sci. Series, 1990, no. 173, pp. 1–34.
Weeraprapan, P., Chantara, S., Kawashima, M., et al., Mouthpart deformities ion non-biting midege lavae from a cadmium contaminated stream in Northern Thailand, Sci. Asia., 2018, vol. 44, pp. 67–73.
Wise, R., Pierstorff, C., Nelson, S., et al., Morphological deformities in Chironomus (Chironomidae: Diptera) larvae as indicators of pollution in lake Winnebago, Wisconsin, J. Great Lakes Res., 2001, vol. 27, pp. 503–509. https://doi.org/503–509. https://doi.org/10.1016/S0380-1330(01)70663-6
Youbi, A., Zerguine, K., Houilia, A., et al., Potential use of morphological deformities in Chironomus (Diptera: Chironomidae) as a bioindicator of heavy metals pollution in North-East Algeria, Environ. Sci. Pollut. Res., 2020, vol. 27, no. 8, pp. 8611–8620.
ACKNOWLEDGMENTS
We were grateful to Dr. Yannawut Uttarak for his support in preparing the map. We are very appreciative of Dr. Adrian R. Plant for his kind help with English editing.
Funding
This research project was financially supported by Mahasarakham University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Abbreviations: BOD5—biochemical oxygen demand; EC—electrical conductivity; DO—dissolved oxygen; PCB—polychlorinated biphenyl; TDS—total dissolved solids.
Rights and permissions
About this article
Cite this article
Thani, I., Prommi, T. Deformation of Mentum Structure in Larvae of Chironomidae (Diptera: Chironomidae) in the Chi River Basin, Thailand. Inland Water Biol 15, 684–692 (2022). https://doi.org/10.1134/S1995082922050091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082922050091