Abstract
Specific features of the formation of the structure of bottom communities in rivers of esker landscapes with dissected topography are analyzed using original samples of macrozoobenthos, and 110 invertebrate taxa are identified. It is shown that the species composition of the macrozoobenthos in rivers on the northern coast of Lake Onega is formed under the effect of the poor regional fauna of Eastern Fennoscandia. The dissected topography is a local factor of the formation of a large number of open lakes in the river network and of many zones under the limnic effect. As a result, benthic communities are characterized by high abundance and biomass (10 000 ind./m2 and 52 g/m2 on average in rapids and 3000 ind./m2 and 12 g/m2 on average in pools, respectively). The portion of collector–filterers in the macrozoobenthos is large (43% of the total biomass on average in the zones of the lake effect), and they are capable of consuming the seston coming from lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
River ecosystems are very closely related to the landscape of the catchment area (Thorp et al., 2006; Allan and Castillo, 2007; Karlsen et al., 2019). The features of climate, topography, vegetation, and human economic activity exert a great impact on the structure and status of river communities, and on that of macrozoobenthos in particular (Heino, 2005; Bogatov and Fedorovskii, 2017; Gerth and Giannico, 2017; Erős and Lowe, 2019). A study of the structure of bottom communities in rivers of various landscapes enables us to reveal the mechanisms of formation and functioning of river ecosystems under the impact of many factors in their catchment areas.
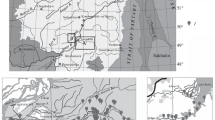
The northern coast of Lake Onega (the area between the Lizhma and Kumsa rivers, including the Zaonezhskii Peninsula) is distinguished by very specific landscapes. The main feature of their topography consists of the spread of narrow and long crystalline ridges (eskers) with altitude reaching 230 m, covered by a thin discontinuous layer of Quaternary deposits. The watersheds in the area are high, and the vertical and horizontal dissection of the surface in strong (Gromtsev and Karpin, 2013). The Zaonezhskii Peninsula is characterized by the mildest climatic conditions for Karelia (the sum of temperatures >10°C reaches ~1500°C, the duration of the frost–free period is 120-130 days, and the period with snow cover lasts 135–145 days) (Gromtsev and Karpin, 2013).
The esker landscape determines a number of hydrological features of rivers. The hydrographic network of the peninsula is characterized by lake–river systems with a linear lacustrine coefficient ≤70% and a stepped longwise section (Litvinenko and Bogdanova, 2013). The dissected and mosaic landscape favors the formation of a wide variety of aquatic biotopes, which can affect the biological diversity and sustainability of communities. Many watercourses are short channels with rapids between lakes, which exert a significant effect on river ecosystems (Baryshev, 2017; Salvo et al., 2020).
It is known that the increased color, low mineralization, and low pH typical for waters of Fennoscandia limit the diversity and abundance of aquatic communities (Tekanova et al., 2019; Kesti et al., 2022). However, waters of the Zaonezh’e are characterized by relatively high mineralization (30–360 mg/L), alkalinity, and trophicity as compared to rivers of the Republic of Karelia in general and by low content of organic substances in lakes and their high content in rivers (Lozovik et al., 2005).
Macrozoobenthos of the Lizhma River, one of the rivers of the northern coast of Lake Onega, is rather comprehensively studied (Khrennikov, 1978; Khrennikov, 2007; Baryshev and Kukharev, 2011). However, there is little information about the bottom communities of other watercourses in this area (Ryabinkin et al., 2000; Komulainen et al., 2013). Understanding the processes of formation of bottom communities in rivers of esker landscapes with high watersheds and pronounced vertical and horizontal dissection is important for revealing the general regularity of the functioning of freshwater ecosystems.
The aim of this work is to analyze the effect of the combination of natural factors related to physicochemical features of the catchment area of rivers in esker landscapes of the northern coast of Lake Onega on the composition, abundance, and trophic structure of macrozoobenthos communities of these rivers.
MATERIALS AND METHODS
The material for the study was collected at 26 stations in ten rivers (Fig. 1) of the northern coast of Lake Onega in 2007–2017. In total, 97 quantitative samples of macrozoobenthos were taken and processed, including 80 from rapids and 17 from pools. Most samples (85) were taken in summer (the second half of July–the first half of August). This season is the most representative for the characteristic of biological aspects of rivers of the Lake Onega basin (Chernov, 1927; Baryshev and Veselov, 2007; Baryshev, 2020). Samples were also taken at three stations in autumn and spring (Table 1) to assess seasonal changes in the trophic structure and abundance of macrozoobenthos.
We examined the main river biotopes: areas of rapids with rocky deposits and pools with soft ground. The thalweg is usually not pronounced on rapids in rivers of Eastern Fennoscandia, so the specification of stations into medial and ripal (Table 1) is performed only for pools.
We took samples by a Surber quantitative frame with a mesh size of 250 µm and an area of 0.04 m2 on stony ground and by a DAK-250 dredger (of 0.25 m2 in area, two lifts per sample) on soft ground (Komulainen et al., 1989). In a laboratory, all invertebrates were removed from the sample under a binocular microscope, counted, and weighed by species. The species whose abundance reached 15% of the total abundance at the station were assigned to dominants by abundance and biomass.
Statistical calculations. The Spearman correlation coefficient and the Kruskal–Wallis criterion were calculated, and samples were tested for the distribution normality (Shapiro–Wilk and Jarque–Bera tests) in the PAST 4.09 program. Data on abundance and biomass were given without standard error due to their asymmetric pattern.
Diversity. The Shannon index (H) is calculated by the formula H = –Ʃ pi ln pi, where pi is the portion of individuals of the ith species in the total number of zoobenthos. The evenness of communities is calculated by the formula E = H/Hmax = H/ln S, where S is the number of species in the community. The Simpson index (D) is chosen as a measure of domination, which is calculated by the formula D = Ʃ pi2, where pi is the portion of individuals of the ith species of the abundance (by the number) of zoobenthos.
Trophic structure. The method of functional groups with respect to nutrition was used to analyze the trophic structure, because they reflect the features of the composition and transformation of organic matter in sections of rivers (Vannote et al., 1980; Minshall et al., 1985). The following groups were identified based on (Merritt et al., 1996; Cummins et al., 2005): shredders, collectors–filterers, collectors–gatherers, predators, and scrapers.
RESULTS
Species composition. The fauna is formed by at least 110 taxa, and most of them are determined to the species level. There are 90 taxa in the benthos of rapids and 45 taxa in the benthos of pools. Data on the occurrence of species and taxa at the stations are given in Table S1.
Communities of rapids are primarily formed by larvae of caddis fly (25 species), mayflies (13 species), stone fly (11 species), and gastropods (seven species). The macrozoobenthos of pools was predominated by chironomids (7), caddis fly (6), bivalves (4), mayflies (4), and Simuliidae (4) with respect to the species number. The occurrence >50% in samples of rapids was detected for representatives of amphibiotic insects: caddis fly Hydropsyche pellucidula (82.5%) and Rhyacophila nubila (62.0%) and Simuliidae spp. (62.5). A high occurrence in samples of bottom communities of pools is typical only for protoaquatic organisms: bivalve mollusks of the genus Euglesa (64.7%) and Sphaerium corneum (29.4%).
Dominant species (Table 2). Chironomid larvae (Procladius sp., Heterotrissocladius marcidus, Procladius convictum gr., Stictochironomus crassiforceps, and Tanytarsus sp) and bivalve mollusks (Euglesa sp.) form the basis of bottom communities of pools. Larvae of caddis fly (Hydropsyche pellucidula, H. siltalai, and Neureclipsis bimaculata), Simuliidae (Simulium sp.), and mayfly (Baetis rhodani) are numerous in communities of rapids.
Abundance of macrozoobenthos. The abundance and biomass of macrozoobenthos varied widely from station to station (Table 3). The median was always lower than the arithmetic mean, which is a consequence of the asymmetric distribution of the abundance (Shitikov et al., 2013). The samplings statistically significantly differed from the normal distribution: the Shapiro–Wilk criterion for the total population was 0.31 (p < 0.001) and the Jarque–Bera test was 16 680 (p < 0.001), while these criteria were 0.49 (p < 0.001) and 3460 (p < 0.001), respectively, for the total biomass.
The comparison of means and medians of the abundance and biomass of macrozoobenthos enables us to conclude that its abundance in rivers of the northern coast of Lake Onega is quite high and exceeds that in other rivers of Eastern Fennoscandia 2–3 times.
The abundance of macrozoobenthos of rapids was significantly higher than that of pools, both in number and in biomass (Table 3). Quantitative parameters of macrozoobenthos depended on the distance from the above-stream lake. For abundance, the Spearman correlation coefficient rs = –0.522 (df = 95, p < 0.001) and the Kruskal–Wallis criterion H (chi2) = 26.17 (p < 0.001). For biomass, rs = –0.701 (df = 95, p < 0.001) and H (chi2) = 47.69 (p < 0.001). The abundance and biomass at different distances from the lake are shown in Fig. 2. The dependence may be described by a power function. The approximation accuracy (R2) is significantly higher for biomass than for abundance, similarly to the Spearman correlation coefficient.
The biomass basis in communities of rapids is formed by larvae of caddis fly, and the abundance is mainly formed by hydroids, caddis fly, and Simuliidae. Chironomid larvae and bivalve mollusks predominate in pool communities (Table 4).
Large bivalve mollusks of the family Unionidae (Anodonta cygnea and Unio pictorum) are revealed in the benthos of four samples taken at three stations (5, 6, and 8) with the occurrence of 4.1% for all samples and 23.5% for samples of the macrozoobenthos in pools. As a result of a large size of individuals, the biomass of these hydrobionts is many times higher than that of other benthic species. The abundance is maximal (120 ind./m2) at station 8, and the biomass is 3600 g/m2.
Trophic structure. The parameters of the trophic structure of macrozoobenthos were calculated by us for three groups of stations: rapids outside the effect of the lake, rapids in the affected zone of the lake (500 m and smaller), and pools (Table 5).
The differences in the trophic structure of macrozoobenthos of the specified biotopes are significant: predators predominate with respect to the role in the total biomass of macrozoobenthos on rapids outside the effect of lakes (the portion of collectors–filterers is also high); collectors–filterers are the dominants in the affected zone of lakes in the communities of rapids, and they form the basis of the biomass of pools.
In rivers of the northern coast of Lake Onega, shredders are represented by caddis fly Potamophylax latipennis, Stenophylax sp., and Ceraclea nigronervosa and stone fly Leuctra fusca and L. digitata. Collectors–filterers are predominated by passive filterers: caddis fly Hydropsyche pellucidula, H. siltalai, Arctopsyche ladogensis, Ceratopsyche newae, C. silfvenii, and Neureclipsis bimaculata and Simuliidae Wilhelmia equina, Odagmia ornata, and Simulium morsitans. Active filterers are represented by small bivalve mollusks of the family Sphaeriidae (Sphaerium corneum) and by species of the genus Euglesa. Some parts of pools are inhabited by large bivalve mollusks of the family Unionidae: Anodonta cygnea and Unio pictorum. Collectors–gatherers are represented by mayfly Baetis rhodani, B. fuscatus, B. vernus, Nigrobaetis digitatus, N. niger, Paraleptophlebia submarginata, etc. This group also includes beetles Elmis aenea and Limnius volckmari; oligochaetes Limnodrilus hoffmeisteri, Lumbriculus variegatus, and Spirosperma ferox; and most species of chironomids. Scrapers are represented by mayfly Heptagenia sulphurea, Serratella ignita, and Ephemerella mucronata, as well as by gastropoda Ampullaceana balthica, Ancylus fluviatilis, Planorbis corneus, Viviparus viviparous, etc. Predators include bugs Aphelocheirus aestivalis; caddis flies Rhyacophila nubila and Rh. fasciata; dragonflies Onychogomphus forcipatus, Gomphus vulgatissimus, and Cordulegaster boltonii; hirudineans Erpobdella octoculata, Glossiphonia complanata, and Helobdella stagnalis; Megaloptera Sialis fuliginosa, S. lutaria, and S. sordida; and Diptera Procladius sp. and Hexatoma sp.
Biological diversity. Parameters of biological diversity are calculated for three groups of stations: rapids beyond the effect of the lake, rapids in the affected zone of the lake (500 m or smaller), and pools (Table 6).
The diversity is the highest for bottom communities of rapids outside the effect of upstream lakes and lowest for the macrozoobenthos of pools.
Seasonal dynamics. The species richness and abundance of macrozoobenthos changes over a year: the quantitative parameters become smaller in summer (Table 7).
The river macrozoobenthos at the lake outlet is characterized by an extremely high abundance (number and biomass) throughout the year. The trophic structure of bottom communities also undergoes seasonal changes. The portion of predators increases and that of gatherers and scrapers decreases in summer (Table 8). The high portion of filterers in the trophic structure indicates a significant effect of the lake, which is seen not only in summer, but also in other seasons.
DISCUSSION
The species composition of the macrozoobenthos in rivers of the northern coast of Lake Onega completely corresponds to the fauna of invertebrates in watercourses of Fennoscandia (Baryshev, 2017). In comparison with streams of other regions, species diversity is low. For example, 123 species were identified when processing only 23 benthic samples from the upper reaches of the Khoper River (Penza oblast) (Silina, 2017). The fauna of the rheophilic freshwater macrozoobenthos of the Caucasus is about 1700 species (Palatov, 2018). In rivers of eastern Sakhalin, 164 species were found (Zhivoglyadova et al., 2012). The poor macrozoobenthos fauna in rivers of the northern coast of Lake Onega is related to rather severe climate of the Republic of Karelia and the high color and low mineralization of waters (Lozovik et al., 2005; Tekanova et al., 2019).
Macrozoobenthos of rapid rivers is characterized by a significant variation in abundance in some sections, which is related to the mosaic pattern of biotopes and sharp changes in hydrological conditions and ground of riverbed (Tiunova, 2006; Zhivoglyadova et al., 2012; Bogatov and Fedorovskii, 2017; Khamenkova and Teslenko, 2021). The abundance in the rivers examined by us also varied ten times (Table 3). However, the mean and median values of the number and biomass obtained in this work for macrozoobenthos of rapids and pools (Table 3, Fig. 2) are rather high for rivers of Eastern Fennoscandia.
It is revealed that the composition of the dominant species, the abundance of macrozoobenthos, and the trophic structure of communities in rivers of the northern coast of Lake Onega are greatly affected by numerous open lakes. A similar dependence is pointed out in (Malmqvist and Eriksson, 2006; Turner et al., 2016). The esker landscape is characterized by a frequent alternation of lake and river fragments of the hydrographic network; thus, a large number of river habitats are located in the zones of limnic effect. This is probably the main reason for the high abundance of the river macrozoobenthos in the studied area in general as compared to other areas of Fennoscandia. Open lakes also favor a high portion of collectors–filterers (consuming limnic seston) in the zone of their impact (Tables 5, 8). It is known that the abundance of macrozoobenthos in the zones where limnic seston enters the river is often increased several times due to collectors–filterers (Valett and Stanford, 2011; Baryshev, 2017). However, in areas beyond the effect of lakes, the relative abundance of collectors–filterers in macrozoobenthos is similar to that in rivers of Fennoscandia and other regions (Tiunova, 2006; Baryshev, 2020). Open lakes exert a negative impact on the parameters of biological diversity (Table 6): they are the highest in communities of rapids at a distance from lakes.
The regularities of seasonal dynamics of macrozoobenthos revealed for the region are seen in rivers of esker landscapes of the northern coast of Lake Onega over a year: a decrease in species richness and abundance in summer due to intensive emergence of amphibiotic insects (Baryshev and Veselov, 2007). Similar regularities are detected for rivers in other regions (Zhivoglyadova et al., 2012; Khamenkova and Teslenko, 2021). The high percentage of lakes in the river network of the studied area results in a stable water regime: moderate floods and a sufficient water amount during low water. This lowers the number and intensity of catastrophic phenomena (such as drying out and sharp fluctuations in the water level, which cause the destruction of bottom communities (Baryshev and Veselov, 2007)).
The structure of river bottom communities of the northern coast of Lake Onega is formed under the effect of local and regional factors. Local ones include the esker landscape with a lot of open lakes. The poor fauna of invertebrates in rivers of Eastern Fennoscandia is a regional factor which determines the species composition of bottom communities of the studied watercourses. There are also general regularities of spatial dynamics of the structure of rheophilic communities in rivers of the northern coast of Lake Onega, the reaction of macrozoobenthos to the input of lake seston in particular.
CONCLUSIONS
The species composition of macrozoobenthos in rivers on the northern coast of Lake Onega is relatively poor despite the dissected topography of the area, various biotopes, relatively favorable climatic conditions, and sufficiently high (for Karelian rivers) water mineralization. The fauna of macrozoobenthos of rivers of this area is primarily determined by the poor species composition of the benthic invertebrates of Eastern Fennoscandia and not by features of the landscape. Macrozoobenthos communities of rivers in this area are characterized by a relatively high biomass and a significant portion of collectors–filterers, which is related to the effect of many open lakes.
REFERENCES
Allan, J.D. and Castillo, M.M., Stream Ecology: Structure and Function of Running Waters, Dordrecht: Springer-Verlag, 2007.
Baryshev, I.A., Taxonomic composition and trophic structure of benthic fauna in rocky rapids and riffles in rivers of the Republic of Karelia and Murmansk Oblast, Inland Water Biol., 2017, vol. 10, no. 4, pp. 405–414. https://doi.org/10.1134/S1995082917040034
Baryshev, I.A., Macrozoobenthos of the rivers of Eastern Fennoscandia, Extended Abstract Doctoral (Biol.) Dissertation, Petrozavodsk: Karel, Nauchn. Tsentr Ross. Akad. Nauk, 2019.
Baryshev, I.A., Zoobenthos of pools of rapid rivers: composition, abundance, and trophic structure (based on the example of eastern Fennoscandia), Inland Water Biol., 2020, vol. 13, no. 1, pp. 69–78.https://doi.org/10.1134/S1995082920010022
Baryshev, I.A. and Kukharev, V.I., Effect of a flowage lake on the structure of zoobenthos in a fast flowing river (case study of the Lizhma River, Onega Lake basin), Uch. Zap. Petrozavodsk. Gos. Univ., 2011, vol. 119, no. 6, pp. 16–19.
Baryshev, I.A. and Veselov, A.E., Seasonal dynamics of benthos and drift invertebrates in some tributaries of lake Onega, Inland Water Biol., 2007, no. 1, pp. 74–80.
Bogatov, V.V. and Fedorovskii, A.S., Osnovy rechnoi gidrologii i gidrobiologii (Fundamentals of River Hydrology and Hydrobiology), Vladivostok: Dal’nauka, 2017.
Chernov, V.K., Results of a hydrobiological study of the Suna, Shuya, Lososinka rivers, and Kosalma channel, Tr. Borodinskoi Biol. Stn., 1927, vol. 5, pp. 190–202.
Cummins, K.W., Merritt, R.W., and Andrade, P.C., The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil, Stud. Neotrop. Fauna Environ., 2005, vol. 40, no. 1, pp. 69–89. https://doi.org/10.1080/01650520400025720
Erős, T. and Lowe, W.H., The Landscape Ecology of Rivers: from Patch-Based to Spatial Network Analyses, Curr. Landscape Ecol. Rep., 2019, vol. 4, pp. 103–112. https://doi.org/10.1007/s40823-019-00044-6
Gerth, W.J., Li, J., and Giannico, G.R., Agricultural land use and macroinvertebrate assemblages in lowland temporary streams of the Willamette Valley, Oregon, USA, Agric. Ecosyst. Environ., 2017, vol. 236, pp. 154–165. https://doi.org/10.1016/j.agee.2016.11.010
Gromtsev, A.N. and Karpin, V.A., 2013. The location of the Zaonezhsky Peninsula in the system of natural zoning and its physical and geographical specifics, Sel’govye landshafty Zaonezhskogo poluostrova: prirodnye osobennosti, istoriya osvoeniya i sokhranenie (Selka landscapes of the Zaonezhskii Peninsula: Natural characteristics, Land Use, Conservation), Petrozavodsk: Karel. Nauchn. Tsentr Ross. Akad. Nauk.
Heino, J., Functional biodiversity of macroinvertebrate assemblages along major ecological gradients of boreal headwater streams, Freshwater Biol., 2005, vol. 50, no. 9, pp. 1578–1587.
Karlsen, C.S., Flindt, M.R., Sønderup, M.J., et al., Impact of land use and runoff on stream quality, Sustainability, 2019, vol. 11, no. 19, art. ID 5479. https://doi.org/10.3390/su11195479
Kesti, P., Hiltunen, M., Strandberg, U., et al., Lake browning impacts community structure and essential fatty acid content of littoral invertebrates in boreal lakes, Hydrobiologia, 2022, vol. 849, pp. 967–984. https://doi.org/10.1007/s10750-021-04760-1
Khamenkova, E.V. and Teslenko, V.A., Structure of macrozoobenthos communities and their biomass dynamics in the Ola river, northern coast of the sea of Okhotsk, Magadan region, Zool. Zh., 2017, vol. 96, no. 6, pp. 619–630. https://doi.org/10.7868/S0044513417060071
Khrennikov, V.V., Benthos in Lake Onega tributaries, in Lososevye Nerestovye Reki Onezhskogo Ozera (Salmon Spawning Rivers of Lake Onega), Leningrad: Nauka, 1978.
Komulainen, S.F., Kruglova, A.N. Khrennikov, V.V., and Shirokov, V.A., Metodicheskie rekomendatsii po izucheniyu gidrobiologicheskogo rezhima malykh rek (Guidelines for the Study of the Hydrobiological Regime of Small Rivers), Petrozavodsk: Karel. Nauchn. Tsentr Ross. Akad. Nauk, 1989.
Komulainen, S.F., Kruglova, A.N., and Baryshev, I.A., et al., Hydrobiological features of reservoirs and streams, in Sel’govye landshafty Zaonezhskogo poluostrova: prirodnye osobennosti, istoriya osvoeniya i sokhranenie (Selka Landscapes of the Zaonezhskii Peninsula: Natural Characteristics, Land Use, Conservation), Petrozavodsk: Karel. Nauchn. Tsentr Ross. Akad. Nauk, 2013.
Litvinenko, A.V. and Bogdanova, M.S., Hydrographic network, in Sel’govye landshafty Zaonezhskogo poluostrova: prirodnye osobennosti, istoriya osvoeniya i sokhranenie (Selka Landscapes of the Zaonezhsky Peninsula: Features of Nature, History of Development, and Conservation), Petrozavodsk: Karel. Nauchn. Tsentr Ross. Akad. Nauk, 2013, p. 36.
Lozovik, P.A., Basov, M.I., and Zobkov, M.B., Surface waters of the Zaonezhskii Peninsula. The chemical composition of water, in Ekologicheskie problemy osvoeniya mestorozhdeniya Srednyaya Padma (Environmental Problems in the Development of the Srednyaya Padva Deposit), Petrozavodsk: Karel. Nauchn. Tsentr Ross. Akad. Nauk, 2005.
Malmqvist, B. and Eriksson, A., Benthic insects in Swedish lake-outlet streams: patterns in species richness and assemblage structure, Freshwater Biol., 2006, vol. 34, no. 2, pp. 285–296. https://doi.org/10.1111/j.1365-2427.1995.tb00888.x
Merritt, R.W., Wallace, J.R., Higgins, M.J., et al., Procedures for the functional analysis of invertebrate communities of the Kissimmee River-floodplain ecosystem, Fla. Sci., 1996, vol. 59, no. 4, p. 216.
Minshall, G.W., Cummins, K.W., Petersen, R.C., et al., Developments in stream ecosystem theory, Can. J. Fish. Aquat. Sci., 1985, vol. 42, pp. 1045–1055.
Palatov, D.M., Rheophilic macrozoobenthos of the Eastern Black Sea region, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Moscow: Mosk. Gos. Univ., 2018.
Ryabinkin, A.V., Kukharev, V.I., and Polyakova, T.N., Macrozoobenthos. Flora and fauna of aquatic ecosystems. Zaonezhje Peninsula, in Inventarizatsiya i izuchenie biologicheskogo raznoobraziya na territorii Zaonezhskogo poluostrova i Severnogo Priladozh’ya (Inventory and Study of Biological Diversity on the Territory of the Zaonezhskii Peninsula and the Northern Ladoga Area), Petrozavodsk: Karel. Nauchn. Tsentr Ross. Akad. Nauk, 2000.
Salvo, J., Valdovinos, C., and Fierro, P., Benthic macroinvertebrate assemblages of a stream-lake network in the upper zone of the trans-Andean basin of the Valdivia River (Chile), N. Z. J. Mar. Freshwater Res., 2020, vol. 55, no. 2, pp. 375–392.
Shitikov, V.K., Rozenberg, G.S., and Zinchenko, T.D., Kolichestvennaya gidroekologiya: metody sistemnoi identifikatsii (Quantitative Hydroecology: Methods of System Identification), Tolyatti: Inst. Ekol. Volzh. Basseina Ross. Akad. Nauk, 2003.
Silina, A.E., Fauna and structure of macrozoobethos communities of the river Khoper under reserve condition, Nauchn. Vedomosti Belgorod. Gos. Univ., Ser.: Estestv. Nauki, 2017, vol. 25, no. 274, p. 59.
Tekanova, E.V., Kalinkina, N.M., and Kravchenko, I.Yu., Geochemical peculiarities of biota functioning in water bodies of Kareliia, Izv. Ross. Akad. Nauk, Ser. Geogr., 2018, no. 1, pp. 90–100. https://doi.org/10.7868/S2587556618010083
Thorp, J.H., Thoms, M.C., and Delong, M.D., The riverine ecosystem synthesis: biocomplexity in river networks across space and time, River Res. Appl., 2006, vol. 22, no. 2, pp. 123–147.
Tiunova, T.M., Trophic structure of invertebrate communities in ecosystems of salmon rivers in the southern Far East, Russ. J. Ecol., 2006, vol. 37, no. 6, pp. 419–425. https://doi.org/10.1134/S1067413606060099
Turner, K.L., Matthews, R.A., and Rawhouser, A.K., Benthic macroinvertebrate assemblages in Kryal and Rhithral Lake outlet streams in the north cascade mountains, Northwest Sci., 2016, vol. 90, no. 2, pp. 206–227. https://doi.org/10.3955/046.090.0211
Valett, H.M. and Stanford, J.A., Food quality and hydropsychid caddisfly density in a lake outlet stream in Glacier national park, Montana, USA, Can. J. Fish. Aquat. Sci., 1987, vol. 44, no. 1, pp. 77. https://doi.org/10.1139/f87-009
Vannote, R.L., Minshall, G.W., Cummins, K.W., et al., The river continuum concept, Can. J. Fish. Aquat. Sci., 1980, vol. 37, no. 1, pp. 130–137.
Zhivoglyadova, L.A., Dairova, D.S., and Labai, V.S., Composition, structure, and seasonal dynamics of macrozoobenthos from the rivers of east Sakhalin, Vseross. Nauchno-Issled. Inst. Rybn. Khoz. Okeanogr., 2012, vol. 171, pp. 199–209.
Funding
This work was supported by the federal budget as part of State Task of the Karelian Scientific Center, Russian Academy of Sciences, theme FMEN-2022-0007.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The author declares that he has no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by I. Bel’chenko
Supplementary Information
Rights and permissions
About this article
Cite this article
Baryshev, I.A. Specific Features of the Composition, Abundance, and Trophic Structure of Macrozoobenthos Communities in Rivers of Esker Landscapes on the Northern Coast of Lake Onega. Inland Water Biol 15, 593–602 (2022). https://doi.org/10.1134/S1995082922050030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082922050030