Abstract
We assessed the efficiency of the transfer of essential substances (carbon, phosphorus, nitrogen, and fatty acids (FA), including polyunsaturated fatty acids (PUFAs)) from phytoplankton to planktonic crustaceans in experimental mesocosms in the presence and absence of fish. The experiments were conducted under mesotrophic conditions in 300 L mesocosms. We have found that transfer efficiencies from producers to consumers are different for different substances. In particular, FA, including PUFAs, are transferred less efficiently than carbon. In contrast, the efficiency of nutrient transfer, especially phosphorus, is higher than that of carbon. This evidences that zooplankton can accumulate nutrients, increasing their quality as a resource for higher trophic levels. Fish significantly reduced the efficiency of carbon transfer from phytoplankton to zooplankton per unit of water volume, but did not affect the transfer of substances per unit of biomass. Thus, the quality of zooplankton as a food resource for higher trophic levels did not decrease in the presence of fish, despite the decline in the efficiency of the transfer of the essential substances per unit of water volume under their influence. Since the efficiency of essential substances transfered from phytoplankton to zooplankton determines the functioning of the entire trophic web, we should seek ways to increase it.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Planktonic communities constitute the basis of the trophic pyramid in freshwater ecosystems. The transfer efficiency of essential substances, including carbon, phosphorus, nitrogen, and FAs (especially PUFAs from phytoplankton to zooplankton), determines the quality of biological resources. In general, only 10% of carbon is transferred from one trophic level to the next (Lindeman, 1942). The efficiency of the transfer of substances can vary from 2–3 to 20% depending on environmental conditions (Slobodkin, 1972). In freshwater lakes, the efficiency of carbon transfer from phytoplankton to zooplankton varies in a wider range, from 5 to 30% (Lacroix, 1999).
For a long time, the flow of matter and energy in the ecosystems was analyzed as a whole without dividing it into components (Pauly and Christensen, 1995; Schulz et al., 2004). However, it has been shown that different substances can be transferred with different efficiencies (Gladyshev et al., 2011) that help zooplankton organisms maintain homeostasis (Sterner and Hessen, 1994). Therefore, zooplankton have to accumulate substances which are in shortage and excrete substances which are in excess, thus maintaining their growth and reproduction rates (Schoo et al., 2013).
One of the factors affecting the efficiency of the transfer of essential substances from phytoplankton to planktonic crustaceans is planktivorous fish. They can have an indirect effect on phytoplankton. Since planktivorous fish reduce planktonic filter feeders, the concentrations of algae increase (Semenchenko et al., 2007). In addition, crustaceans can consume a narrow size-range of food particles, usually <30 μm (Sommer and Sommer, 2006); therefore, fish can increase the proportion of small algae species by reducing the number of crustaceans. They are also able to facilitate cyanobacteria development, as was shown in a eutrophic lake (Kolmakov and Gladyshev, 2003; Kolmakov, 2014) and experimental conditions (Feniova et al., 2019). It is noteworthy that the composition of essential substances is not identical in different algae species (Gulati and DeMott, 1997; Müller-Navarra, 1995). Thus, potential changes in the species and size structure of phytoplankton communities caused by fish can alter the contents of essential substances in the food resources for planktonic crustaceans, which in turn can affect the efficiency of transfer of essential substances from phytoplankton to zooplankton, measured as a ratio of secondary production/primary production.
The goal of the study was to assess the transfer efficiency of essential substances (carbon; phosphorus; nitrogen; and FAs, including PUFAs) from phytoplankton to planktonic crustaceans in mesotrophic conditions. The studies were conducted in experimental mesocosms in which we manipulated the presence and absence of fish to determine their effect on the structure of phytoplankton and, as a result, on the efficiency of transfer of essential substances.
MATERIALS AND METHODS
The studies were conducted in the 300-L tanks (0.94 × 0.64 × 0.50 m), which were filled with water from the mesotrophic Lake Majcz (northeastern Poland, area 163.5 ha, maximum depth 16.4 m, and average depth 6 m) (Gliwicz et al., 1981). The experiment consisted of two treatments—the control (C), where fish was absent, and fish treatment (F). Each treatment was replicated in triplicate mesocosms. In fish mesocosms, one ruff Gymnocephalus cernuus (L., 1758) of 7.5–11 cm was added. Fish were kept in 5-L boxes suspended in the mesocosms. The boxes had large slots that allowed zooplankton to pass freely through, but kept the fish inside. The fish were let out of the cage for only 1 h each day to feed freely. Thus, we limited the predation of fish on zooplankton to prevent them from overexploitation by fish. The experiment was conducted for 30 days.
Сhlorophyll a was recorded every 10 days using a FluoroProbe spectrophotometer, which measured the concentrations of total chlorophyll and chlorophyll of green algae, cryptophytes, diatoms, and cyanobacteria. Phytoplankton biomass (mg C/L) was found based on the total chlorophyll a concentration (μg/L) using the equations after Yacobi and Zohary (2010).
For a more detailed analysis of the taxonomic structure of phytoplankton in the experiment, the microscopic method was used. Phytoplankton were concentrated by settling and preserved with a Utremel solution and 4% formaldehyde (Kuzmin, 1975). Algal cells were counted under a light microscope (Nikon Optiphot 2). The biomass of individual species or genera (mg/L) was determined based on their cell sizes and approximations to simple geometric shapes (Vinberg and Lavrenteva, 1982; Mikheeva, 1989). Algal cell sizes were measured using an ocular micrometer. The phytoplankton size structure was represented by three fractions (ranges): <30, 30–60, and >60 μm.
Samples of planktonic crustaceans were collected by a 2.6-L Limnos sampler every 10 days and fixed with 4% formaldehyde. The wet weight of crustaceans was estimated by applying the relationships between the body mass of planktonic crustaceans and their average length (Błędzki and Rybak, 2016). To convert the wet weight of planktonic crustaceans in carbon units, we used the conversion coefficient proposed by Alimov (1989).
We collected seston and zooplankton for content analyses of FA, PUFA (EPA (20:5n-3) and DHA (22:6n-3)), carbon, phosphorus, and nitrogen on the first and final days of the experiment not to disturb the experimental conditions, since these samples required up to 100 L of water. Samples for seston analysis were preliminarily passed through a 110-µm mesh sieve to remove large zooplankton and/or other particles, and then they were filtered onto precombusted glass–fiber GF/F filters (Whatman, United States) until there was intensive coloring of the filter. Then the filters for FA analysis were dried for 30 min, transferred into a chloroform : methanol (2 : 1) solution, and kept at a temperature of –20°С. The filters for carbon, phosphorus, and nitrogen contents in seston were dried at ambient temperature overnight and stored dry in a desiccator until further analyses.
Samples of water for contents of FA, PUFA, carbon, phosphorus, and nitrogen in planktonic crustaceans were passed through a 110-µm-mesh sieve. Then, the zooplankton left on the sieve was dried with the filter paper and weighed. Samples for FA were kept in a chloroform–methanol solution at –20°С. Samples for carbon, phosphorus, and nitrogen were dried at 75°C overnight and then stored in a desiccator for further analysis.
FAs and PUFAs were measured by a gas chromatograph with a mass spectrometer detector (model 6890/5975С, Agilent, United States) (Kalachova, 2011). Organic carbon and nitrogen were measured using a Flash EA 1112 NC Soil/MAS 200 elemental analyzer (ThermoQuest, Milan, Italy) (Gladyshev et al., 2007). The content of particulate total phosphorus was estimated following the conventional photocolorimetric method (Murphy and Riley, 1962).
We calculated PP based on chlorophyll a concentrations and the potential photosynthetic capacity of phytoplankton measured by polychromatic diuron-induced fluorescence method (DCMU-fluorescence, 3-(3,4-dichlorophenil)-1,1-dimethylurea) using a FluoroProbe spectrophotometer FluoroProbe. This method is described in detail elsewhere (Gaevsky et al., 2005, Kolmakov et al., 2008).
SP were calculated using regression models of Stockwell and Johannsson (1997):
where P is daily production (μg, dry weight L–1 Day–1), M is average body mass (μg), and N is number of individuals L–1.
The efficiency of carbon transfer from producers to consumers per unit of water volume is determined as the ratio secondary production/primary production expressed in percentage (Gladyshev et al., 2011). If productions are calculated per unit of volume (L), they characterize the efficiency of transfer of substances in a water body (in our case, in mesocosm). The productions per unit of biomass are estimated as PP/Bphyto or SP/Bcrust, while the efficiency of carbon transfer from phytoplankton to planktonic crustaceans per unit of biomass is determined by the ratio SP/Bcrust : PP/Bphyto (%). Since this ratio shows the efficiency of transfer of the substances per unit of biomass, it is not dependent on the concentrations of planktonic organisms.
The rates of accumulation of nitrogen (AN) and phosphorus (AP) by phytoplankton and planktonic crustaceans per unit of water volume and per unit of biomass were calculated as follows:
where А is the rate of accumulation per unit of volume, A' is the rate of accumulation per unit of biomass, P/N is phosphorus/nitrogen, phyto is phytoplankton, and crust is planktonic crustaceans; units: Р/N : C, mg/mg of carbon; SP and PP, mg С L–1 day–1; Bphyto and Bcrust, mg С/L.
The efficiency of nitrogen and phosphorus transfer from seston to planktonic crustaceans per unit of volume and per unit of biomass was calculated as follows:
For convenience, the equations for calculating productions and transfer efficiencies of nutrients and their units are presented in Tables 1 and 2.
The productions of PUFAs (EPA, DHA) and FAs of phytoplankton and planktonic crustaceans, as well as their transfer efficiencies from phytoplankton to planktonic crustaceans per unit of volume and per unit of biomass, were calculated using similar ratios, which were applied for nutrients. The equations of the productions and transfer efficiencies for FAs, including PUFAs, and units for each parameter are presented in Tables 1 and 2.
Statistical analysis of the data was conducted using Past 3.20. The data were examined for normal distribution by the Shapiro–Wilk test. The concentrations of chlorophyll a; biomasses of the size groups of algae and biomasses of planktonic crustaceans; and PP, SP, and P/B coefficients were compared between the treatments using Student’s t-test; in the absence of normal distribution, we used the nonparametric Mann–Whitney U-test. To compare the accumulations of nitrogen and phosphorus, as well as the transfer efficiencies of FAs, including PUFAs, between the experimental treatments, one-way analysis of variance was used (Tukey test). The starting data were excluded from statistical analyses, because there were no effects of fish on the parameters of plankton communities on the first day of the experiment.
RESULTS
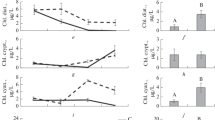
The concentrations of the total chlorophyll and chlorophyll of diatoms were significantly higher in the fish treatment relative to the control (t = 2.8, p = 0.01; t = 4.4, p < 0.01, respectively) (Fig. 1).
Dynamics of chlorophyll of greens (a), diatoms (c), cryptophytes (e), cyanobacteria (g) and total chlorophyll (i), (±SE), and comparison of the means between the experimental treatments (b, d, f, h, j). Here and in Figs. 2‒6, C is control and F is fish treatment. Letters a and b indicate significant differences at p < 0.05 (Student’s t-test).
At the beginning of the experiment, the dominating taxa were dinoflagellates Peridinium cinctum (O.F. Müller) Ehrenberg and chrysophytes Dinobryon bavaricum Imhof and Chrysidalis peritaphrena J. Schiller (Fig. 2). On day 10, in both treatments, in addition to chrysophytes and dinoflagellates, the diatom Fragilaria sp. appeared. By day 20 and day 30 in both treatments, the domination in phytoplankton had shifted to the greens (Oedogonium sp., Mougeotia sp.) and diatoms (Ulnaria acus (Kützing) Aboal, Fragilaria crotonensis Kitton, Cyclotella sp.). There were no significant differences in the biomass of the individual taxa of phytoplankton between the control and fish treatment (t-test, p > 0.05). In the course of the experiment, the structure of phytoplankton was gradually shifting from chrysophytes and dinoflagellates towards greens and diatoms in both treatments. The biomass of all three sizes of algae fractions did not differ between the fish treatment and the control (t-test, p > 0.05). In the experiment, the large size fraction >60 μm was the most abundant; at the end of the experiment, the intermediate size fraction, 30–60 μm, was absent (Fig. 3).
The biomasses of cladocerans were significantly higher in the control than in the fish treatment (t = 3.3, p < 0.01) (Fig. 4). There were no statistically significant differences in the mean biomasses of copepods between the treatments (р > 0.05).
The fish did not affect PP (p > 0.05) (Fig. 5). SP was significantly lower in the fish treatment than in the control (t = 3.2, p = 0.01). As a result, carbon was transferred from phytoplankton to planktonic crustaceans in the fish treatment less efficiently than in the control; on average, its transfer efficiency per unit of water volume reached 4 and 14%, respectively (t = 2.6, p = 0.02).
In the fish treatment, the ratio of the production of crustaceans to their biomass increased relative to the control (U = 18, p = 0.05) (Fig. 6). The efficiencies of carbon transfer from phytoplankton to crustaceans per unit of biomass did not significantly differ between the treatments; on average, in the control and fish treatments, they were 27 and 35%, respectively.
The rates of accumulation of nitrogen and phosphorus in the phytoplankton and planktonic crustaceans were similar in both experimental treatments (Table 1). Therefore, the transfer efficiencies of nitrogen and phosphorus from phytoplankton to planktonic crustaceans did not differ between the treatments either per unit of volume or per unit of biomass. In the control and fish treatment, the efficiencies of nitrogen transfer per unit of volume were, on average, 4 and 5%, respectively. The efficiencies of its transfer per unit of biomass were accordingly 48 and 31%. The phosphorus transfer efficiencies were higher, 9 and 17% per unit of volume, and 68 and 114% per unit of biomass in the control and fish treatment, respectively. Thus, phosphorus per unit of biomass was more efficiently transferred from phytoplankton to planktonic crustaceans than carbon.
PUFAs and FAs were less efficiently transferred from phytoplankton to planktonic crustaceans than carbon, nitrogen, and phosphorus (Table 2). The transfer efficiency of EPA per unit of volume was <1%, while per unit of biomass it reached 2–3%. The transfer efficiency of DHA was 1–2% per unit of volume and 5–12% per unit of biomass. The transfer efficiencies of FAs accounted for <1% and slightly >1% per unit of volume and per unit of biomass respectively. There were no differences between the transfer efficiencies of PUFAs and FAs in the control and fish treatment.
DISCUSSION
Besides organic carbon, phytoplankton produce essential polyunsaturated fatty acids, including EPA and DHA. These valuable molecules are produced mainly by specific species of microalgae, which transfer them to consumers, and further they are transported along trophic chains to higher trophic levels, including humans (Uttaro, 2006; Lands, 2009). Phytoplankton serves also as a source of phosphorus and nitrogen for higher levels of the trophic webs. Algae do not produce nutrients, but convert them from dissolved inorganic compounds into organic forms, which can be accumulated by animals. The more efficiently phytoplankton transfer essential substances to zooplankton, the more valuable zooplankton is as a food resource for higher trophic levels.
The carbon transfer efficiency in the fish treatment did not exceed 4% while, in the control, this parameter reached 14%. This difference was attributed to the fact that there were more cladocerans in the control than in the fish treatment. There were no differences in the biomass of copepods between the experimental treatments. Concentrations of PUFAs, phosphorus, and nitrogen per unit of volume did not differ between the control and fish treatment, despite the lower transfer efficiency of total organic carbon in the fish treatment than in the control. Therefore, the transfer efficiency of these substances was not closely related to carbon transfer.
Our data showed that different substances were transferred with different efficiencies. The efficiencies of phosphorus transfer from phytoplankton to zooplankton were higher than those of the other substances and varied in the range of 9–17%, nitrogen transfer efficiency was 4–5%, PUFA efficiencies were 2%, and FAs were transferred with efficiencies of <1%.
The transfer efficiencies of carbon and essential substances per unit of water volume are determined by the ability of planktonic crustaceans to convert consumed particles into biomass and the concentration of phytoplankton and crustaceans. The content of the consumed substances in the biomass is measured as a ratio of production to biomass (P/B coefficient). P/B coefficient is regarded as the basis of the production potential and can be compared between communities with different densities (Vinberg, 1968; Ikeda et al., 2002). The efficiencies of carbon transfer (SP/B : PP/B) from phytoplankton to planktonic crustaceans per unit of biomass reached 27 and 35% in the control and fish treatment respectively, but did not significantly differ between the experimental treatments. DHA was transferred more efficiently than the other fatty acids (4–12%), EPA was transferred with an efficiency of 2–3%, and FA efficiency was 1%. Phosphorus and nitrogen were transferred with greater efficiency, i.e., 68–113 and 31–48%, respectively. Thus, the efficiencies of nitrogen and phosphorus transfer from primary producers to crustaceans were higher than the carbon-transfer efficiency, while the transfer efficiencies of FA, including PUFA, on the contrary, were lower relative to carbon.
The results of our work are consistent with the findings of other researchers. For example, Hudson et al. (1999) indicated that zooplankton is an important source of nutrients. Karpowicz et al. (2019) experimentally showed that crustaceans can concentrate phosphorus in their bodies; therefore, the content of phosphorus in zooplankton can be higher than in phytoplankton. Previously, we found that, under mesotrophic conditions, the phosphorus content in planktonic crustaceans was 3–4 times more than in seston, while the contents of PUFAs and FAs in crustaceans, on the contrary, were significantly lower than in seston (Feniova et al., 2019). Thus, some substances can accumulate in biomass; others can be expended in the metabolism, or be assimilated poorly because their content in the edible algae fractions (<30, 30–60 μm) can be smaller than in inedible fraction (>100 μm).
Short-chained saturated FAs were transferred less efficiently than PUFAs. Similar results were obtained by Gladyshev et al. (2011). To maintain a high content of PUFAs, cladocerans mainly use other FAs as an energy source, catalyzing them actively (Brett et al., 2006). We expected that the transfer efficiencies of the essential PUFAs from phytoplankton to crustaceans would exceed that of carbon, but their transfer efficiencies were lower. This was attributed to the fact that large diatoms and green algae, whose sizes exceeded the spectrum of edible particles, were dominant in the experiment. In addition, only certain groups of microalgae can effectively synthesize EPA and DHA, namely diatoms, cryptophytes and dinophytes (Cohen et al., 1995; Harwood, 1996; Heinz, 1993; Tocher et al., 1988). By day 30 of the experiment, the planktonic algae were gradually being displaced by periphyton species, which were mostly represented by attached green algae, which worsened the quality of algae community as a food resource.
We suggest that the efficiency of the PUFA transfer can vary depending on the composition of phytoplankton. Therefore, this parameter may be higher (Gladyshev et al., 2011) in particular conditions and lower in other conditions than the carbon transfer efficiency (our experiments). The quality of zooplankton as a resource for higher trophic levels will depend on how efficiently PUFAs are transported. In the experiment, FAs, including PUFAs, were transferred less efficiently than carbon. In contrast, the efficiencies of nutrient transfer, especially that of phosphorus, were greater than in carbon. This indicates that planktonic crustaceans act as a sink of nutrient accumulation, supplying higher trophic levels with organic matter enriched by nutrients. Fish significantly reduced the efficiency of carbon transfer from phytoplankton to crustaceans per unit of volume, but they did not affect the transfer of essential substances per unit of biomass; i.e., the fish did not affect the quality of planktonic crustaceans as a food resource under mesotrophic conditions. Since the efficiency of the transfer of essential substances from phytoplankton to zooplankton determines the functioning of the whole trophic chain, we should seek approaches to increase it.
CONCLUSIONS
Fish reduced efficiencies of carbon transfer from phytoplankton to crustaceans per unit of water volume. The transfer efficiency of the other essential substances did not decrease relative to the control. Phosphorus was transferred more efficiently than carbon due to its ability to accumulate in crustacean bodies. FAs, including PUFAs, were transported less efficiently than carbon because food resources were mainly represented by inedible colonial forms (>50% of the total biomass). Among PUFAs, DHA was transferred more efficiently than EPA. FAs were transferred less efficiently than PUFAs. This difference in efficiencies was likely attributed to the fact that short-chained saturated FAs mainly serve as energy sources.
REFERENCES
Alimov, A.F., An Introduction to Production Hydrobiology, Leningrad: Gidrometeoizdat, 1989.
Błędzki, L.A. and Rybak, J.I., Freshwater Crustacean Zooplankton of Europe, Basel, Switzerland: Springer, 2016. https://doi.org/10.1007/978-3-319-29871
Brett, M.T., Muller-Navarra, D.C., Ballantyne, A.P., et al., Daphnia fatty acid composition reflects that of their diet, Limnol. Oceanogr., 2006, vol. 51, p. 2428.
Cohen, Z., Norman, H.A., and Heimer, Y.M., Microalgae as a source of n-3 fatty acids, in Plants in Human Nutrition. World Review of Nutrition and Dietetics, Basel: Karger, 1995, vol. 77, p. 1.
Feniova, I., Sakharova, E., Karpowicz, M., et al., Direct and indirect impacts of fish on crustacean zooplankton in experimental mesocosms, Water, 2019, vol. 11, no. 10. https://doi.org/10.3390/w11102090
Gaevsky, N.A., Kolmakov, V.I., Anishchenko, O.V., and Gorbaneva, T.B., Using DCMU— fluorescence method for identification of dominating phytoplankton groups, J. Appl. Phycol., 2005, vol. 17, p. 483.
Gladyshev, M.I., Sushchik, N.N., Kolmakova, A.A., et al., Seasonal correlations of elemental and ω3 PUFA composition of seston and dominant phytoplankton species in a eutrophic Siberian Reservoir, Aquat. Ecol., 2007, vol. 41, p. 9. https://doi.org/10.1007/s10452-006-9040-8
Gladyshev, M.I., Sushchik, N.N., Anishchenko, O.V., et al., Efficiency of transfer of essential polyunsaturated fatty acids versus organic carbon from producers to consumers in a eutrophic reservoir, Oecologia, 2011, vol. 165, p. 5211. https://doi.org/10.1007/s00442-010-1843-6
Gliwicz, Z.M., Ghilarov, A., and Pijanowska, J., Food and predation as major factors limiting two natural populations of Daphnia cucullata Sars, Hydrobiologia, 1981, vol. 80, p. 205. https://doi.org/10.1007/BF00018359
Gulati, R. and DeMott, W., The role of food quality for zooplankton: remarks on the state-of-the-art, perspectives and priorities, Freshwater Biol., 1997, vol. 38, pp. 753–768.
Harwood, J.L., Recent advances in the biosynthesis of plant fatty acids, Biochim. Biophys. Acta, 1996, vol. 1301, p. 7.
Heinz, E., Biosynthesis of polyunsaturated fatty acids, in Lipid Metabolism in Plants, Boca Raton: CRC Press, 1993, p. 34.
Hudson, J.J., Taylor, W.D., and Schindler, D.W., planktonic nutrient regeneration and cycling efficiency in temperate lakes, Nature, 1999, vol. 400, p. 659. https://doi.org/10.1038/23240
Ikeda, T., Hirakawa, K., and Shiga, N., Production, metabolism and production/biomass (P/B) ratio of Metridia pacifica (Crustacea; Copepoda) in Toyama Bay, southern Japan Sea, Plankton Biol. Ecol., 2002, vol. 49, no. 2, p. 58.
Kalachova, G.S., Gladyshev, M.I., Sushchik, N.N., and Makhutova, O.N., Water moss as a food item of the zoobenthos in the Yenisei River, Centr. Eur. J. Biol., 2011, vol. 6, p. 236. https://doi.org/10.2478/s11535-010-0115-0
Karpowicz, M., Feniova, I., Gladyshev, M.I., et al., The stoichiometric ratios (C:N:P) in a pelagic food web under experimental conditions, Limnologica, 2019, vol. 77, p. 125690. https://doi.org/10.1016/j.limno.2019.125690
Kolmakov, V.I., Role of Microcystis aeruginosa passing through the digestive tracts of filter-feeding animals in eutrophic water reservoirs (review), Contemp. Probl. Ecol., 2014, vol. 7, p. 455. https://doi.org/10.1134/S1995425514040052
Kolmakov, V.I. and Gladyshev, M.I., Growth and potential photosynthesis of cyanobacteria are stimulated by viable gut passage in crucian carp, Aquat. Ecol., 2003, vol. 37, p. 237.
Kolmakov, V.I., Anishchenko, O.V., Ivanova, E.A., et al., Estimation of periphytic microalgae gross primary production with DCMU-fluorescence method in Yenisei River (Siberia, Russia), J. Appl. Phycol., 2008, vol. 20, pp. 289–297. https://doi.org/10.1007/s10811-007-9246-8
Kuz’min, G.V., Phytoplankton, in Metodika izucheniya biogeotsenoza vnutrennikh vodoemov (Methods for Studying the Biogeocenosis of Inland Water Bodies), Moscow: Nauka, 1975, p. 73.
Lacroix, G., Biomass and production of plankton in shallow and deep lakes: are there general patterns?, Ann. Limnol., 1999, vol. 35, no. 2, p. 111.
Lands, W.E.M., Human life: caught in the food web, in Lipids in Aquatic Ecosystems, New York: Springer, 2009, p. 327.
Lindeman, R.L., The trophic-dynamic aspect of ecology, Ecology, 1942, vol. 23, p. 399.
Mikheeva, T.M., Methods of quantitative estimates of nanophytoplankton (review), Hydrobiol. J., 1989, vol. 25, p. 3.
Müller-Navarra, D.C., Evidence that a highly unsaturated fatty acid limits daphnia growth in nature, Arch. Hydrobiol., 1995, vol. 132, pp. 297–307.
Murphy, J. and Riley, J.P., A modified single solution method for the determination of phosphate in natural waters, Anal. Chim. Acta, 1962, vol. 27, p. 31.
Pauly, D. and Christensen, V., Primary production required to sustain global fisheries, Nature, 1995, vol. 374, p. 255.
Schoo, K.L., Malzahn, A.M., Krause, E., and Boersma, M., Increased carbon dioxide availability alters phytoplankton stoichiometry and affects carbon cycling and growth of a marine planktonic herbivore, Mar. Biol. (Berlin), 2013, vol. 160, p. 2145. https://doi.org/10.1007/s00227-012-2121-4
Schulz, M., Koschel, R., Reese, C., and Mehner, T., Pelagic trophic transfer efficiency in an oligotrophic, dimictic deep lake (Lake Stechlin, Germany) and its relation to fisheries yield, Limnologica, 2004, vol. 34, p. 264.
Semenchenko, V.P., Razlutskij, V.I., Feniova, I.Yu., and Aibulatov, D.N., Biotic relations affecting species structure in zooplankton communities, Hydrobiologia, 2007, vol. 579, no. 1, p. 219. https://doi.org/10.1007/s10750-006-0411-x
Slobodkin, L.B., On the inconstancy of ecological efficiency and the form of ecological theories, in Growth by Intussusception: Ecological Essays in Honor of G. Evelyn Hutchinson, Hamden, CT: Archon Books, 1972.
Sommer, U. and Sommer, F., Cladocerans versus copepods: the cause of contrasting top-down controls on freshwater and marine phytoplankton, Oecologia, 2006, vol. 147, p. 183. https://doi.org/10.1007/s00442-005-0320-0
Sterner, R.W. and Hessen, D.O., Algal nutrient limitation and the nutrition of aquatic herbivores, Annu. Rev. Ecol. Syst., 1994, vol. 25, p. 1.
Stockwell, J.D. and Johannsson, O.E., Temperature-dependent allometric models to estimate zooplankton production in temperate freshwater lakes, Can. J. Fish. Aquat. Sci., 1997, vol. 54, p. 2350. https://doi.org/10.1139/f97-141
Tocher, D.R., Sargent, J.R., and Frerichs, G.N., The fatty acid compositions of established fish cell lines after long-term culture in mammalian sera, Fish Physiol. Biochem., 1988, vol. 5, p. 219.
Uttaro, A.D., Biosynthesis of polyunsaturated fatty acids in lower eukaryotes, IUBMB Life, 2006, vol. 58, p. 563. https://doi.org/10.1080/15216540600920899
Vinberg, G.G., Metody opredeleniya produktsii presnovodnykh zhivotnykh (Methods for Determining the Production of Freshwater Animals), Minsk: Vysheishaya shkola, 1968.
Vinberg, G.G. and Lavrenteva, G.M., Guidelines for the collection and processing of materials for hydrobiological studies in freshwater bodies, in Phytoplankton and Its Products, Leningrad: Gosniorkh USSR, 1982, p. 1.
Yacobi, Y.Z. and Zohary, T., Carbon: chlorophyll a ratio, assimilation numbers and turnover times of Lake Kinneret phytoplankton, Hydrobiologia, 2010, vol. 639, p. 185. https://doi.org/10.1007/s10750-009-0023-3
Funding
The collection of biological material and experimental studies were supported by the Polish National Science Centre (project no. UMO-506 2016/21/B/NZ8/00434). Phytoplankton samples were processed with support from the Russian Foundation for Basic Research (project no. 18-54-00002 Bel_a), zooplankton samples were processed and analyzed using the Belarusian Republican Foundation for Fundamental Research (project no. BRFFR no. B18R-004), and statistical data analyses were conducted as part of State Task no. AAAA-A18-118012690096-1. The interpretation of the results, review of the literature, and preparation of materials for publication were funded by the Russian Science Foundation (project no. 16-14-10323).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by I. Feniova
Abbreviations: Bcrust, crustacean biomass; Bphyto, phytoplankton biomass; SP, secondary production (crustacean production); DHA, docosahexaenoic acid; FAs, fatty acids; PUFAs, polyunsaturated fatty acids; PP, primary production; and EPA, eicosapentaenoic acid.
Rights and permissions
About this article
Cite this article
Feniova, I.Y., Sakharova, E.G., Buseva, Z.F. et al. Efficiency of Transfer of Essential Substances from Phytoplankton to Planktonic Crustaceans in Mesotrophic Conditions. Inland Water Biol 14, 49–59 (2021). https://doi.org/10.1134/S1995082920040033
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995082920040033