Abstract
Data on the first recorded marine alga Prorocentrum leve Faust, Vandersea, Kibler, Tester & Litaker (Dinophyceae) in a Russian inland waterbody, the hypersaline Lake Manych-Gudilo, are given. As a result, in May, July, and October 2016 and February 2017 it was revealed that the species was found in small quantities, mainly in the northwestern part of the lake. Its morphology, studied with an electron scanning microscope, is largely consistent with that described in the literature. The presence of marine species P. leve can serve as indirect evidence of the fragmentation of marine algal areas as a result of paleobasin regression. The results expand information on the composition of the algal flora of inland water bodies in Russia, as well as on the environmental opportunities and general distribution of P. leve.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Dinophyte algae (Dinophyceae) or dinoflagellates are one of the most diverse and widespread microalgae groups in the world’s oceans. There are now 4000 known fossils and >2500 reference dinoflagellates, 90% of which live in seas and the rest in freshwater [17]. However, the study of continental hypersaline reservoirs is no less relevant, and data on the dinoflagellates of such reservoirs in southern European Russia and adjacent territories are extremely sporadic [2, 12, 24].
The greatest theoretical interest, especially in terms of florogenetic and palaeogeographic reconstructions, is the data on dinoflagellates of the continental reservoirs of the Ponto-Caspian Sea, which are historically connected with the epicontinental seas, which are the remnants of the ancient ocean of Eastern Paratetetis. One such reservoir is the hypersaline Lake Manych-Gudilo located in the central part of the Kumo-Manych depression, the occurrence of which is historically connected with the existence of sea straits (the last one is the Khvalynsky strait) in the late Pleistocene. The final isolation of the reservoir from sea basins with a developed river network was established in the late Holocene [15]. Fauna and flora of water bodies of the Manych depression are extremely heterogeneous, which is connected not only with their subsequent fragmentation in the late Pleistocene and Holocene, but also with modern human activity—the construction of a cascade of reservoirs and irrigation canals, as well as the introduction of commercial species of hydrobionts [7]. The existence of numerous fossil Mediterranean or Caspian molluscs, benthic, and planktonic foraminifera is evidence of the existence of at least four differently directed sea straits in the Manych depression [11, 15]. Unlike many benthic organisms, on the basis of which the paleontological chronicle of the Ponto-Caspian Sea is based, the presence of sea-based microalgae common to the Azov and Black Seas in the Manych depression reservoirs [5] can serve as indirect evidence of the fragmentation of their ranges as a result of paleobasin regression.
One such example is the detection of a seaweed of the genus Prorocentrum in Lake Manych-Gudilo, morphologically fully corresponding to the species P. leve Faust, Vandersea, Kibler, Tester & Litaker, recently described (as P. levis Faust, Vandersea, Kibler, Tester & Litaker) from the Caribbean Sea [22]. The genus Prorocentrum Ehrenberg was singled out in 1834 [21] and currently has ~80 species [26]; its representatives are mainly marine plankton or benthic inhabitants, except for two species found in fresh water [25]. Ten species of the genus are known to be potentially toxic [25, 28]. The species P. leve, collected and described from a floating detritus off the coast of Belize in the Caribbean Sea, has also been shown to be capable of producing okadaic acid and dinophysistoxin-2 [22]. This species was later found in the northern part of the Aegean Sea, western and northwestern Adriatic Sea [18, 27, 29], and the southeastern part of the Bay of Biscay [19], but tests of these strains of P. leve did not confirm the presence of toxicity [18, 19, 29].
As previous studies [4, 7, 10, 16] have shown, Lake Manych-Gudilo is characterized by a change in the taxonomic composition of phytoplankton depending on the transformation of its hydrochemical regime. During the period of the greatest flooding, when the level of water salinity did not exceed 20 g/L, only one type of dinophyte algae Gymnodinium sp. has been noted [4]. With the subsequent increase in the salinity of the lake, the composition of dinophyte species has increased to seven species, which are typical mainly for marine reservoirs [5–7, 16].
In Lake Manych-Gudilo, P. leve was found for the first time in fixed samples in 2016 in the course of comprehensive monitoring studies of water bodies of the Lake Manych-Gudilo depression.
The goal of this work was to describe the morphology of P. leve in a hypersaline continental waterbody (using light and scanning electron microscopy) and its occurrence in this area.
MATERIALS AND METHODS
Physical and geographical characteristics and hydrochemical regime of the reservoir. Lake Manych-Gudilo is an inland shallow-water lake (average depth of 2.6 m), a hypersaline body of water located in the zone of unstable and insufficient humidification of the temperate climatic zone [7, 14]. The low rainfall, high summer temperatures, and prolonged hot dry winds in the region result in high evaporation rates, leading to an even higher salinity of the lake. Mineralization of this reservoir also depends on the volume of fresh water supplying it to the Bolshoy Egorlyk and Kalaus rivers [7, 9, 14]. At present, the total salinity of Lake Manych-Gudilo varies from 5.2 (at the river mouths) to ≥50 g/L. The lake waters are considered meso- and polyhalic and, in terms of the ratio of basic saline ions, they occupy an intermediate position between the carbonate−sulfate−calcium type of continental saline waters and the sodium chloride type of sea waters [9, 14].
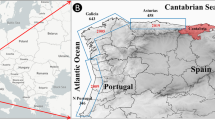
Methods of investigation. In the course of the expedition studies at the reservoirs of the Manich system, the staffs of the Southern Scientific Center of the Russian Academy of Sciences (RAS) in 2016 took water samples in May at five stations, in July at three stations, and in October at 13 stations (from the surface horizon). In addition, in February 2017, subglacial water samples and aquatic vegetation residues deposited on the seabed were collected at two stations (Fig. 1).
To study the morphological features of organisms, we used light microscopy (microscope Mikmed-5), epiluminescence (fluorescent microscope Mikmed-2-Var-11), and scanning electron microscopy (SEM EVO-40 XVP). Water samples were fixed with an acid solution of Lugol and 25% glutaric aldehyde with a final concentration in the sample of 1% [13].
For electron microscopy, algae cells were deposited on membrane nuclear filters with a pore diameter of 1 µm, washed twice with distilled water, dehydrated through a series of ascending concentration ethanol solutions (25, 40, 55, 70, and 96%), and dried in air at room temperature. The filters were then attached to the microscopic tables with a double-sided conductive carbon tape. Surface spraying of the samples was carried out with the help of Au/Pd-target in the Mini Sputter Coater SC7620 (Quorum Technologies) vacuum spraying unit.
The cells (24 examples) and their details were measured using electronic microphotographs using the Scandium software (v. 5.0), as well as the light microscope and ToupCam UCMOS 10 MP video eyepiece. The standard deviation is given as an indicator of the variation of characters. To identify the microalgae’s calcareous plates, we used the terminology proposed by Hoppenrat et al. [25].
RESULTS
Morphology of Prorocentrum leve. These are cells of highly oval shape, symmetrical, and concave/double concave from the side of the belt (Figs. 2a−2g). Cell length is 43.74−50.37 µm (47.99 ± 1.52 µm), width is 39.73−45.31 µm (42.85 ± 1.38 µm), and the length to width ratio is 1.05−1.18 (1.12 ± 0.03). The cells contain chloroplasts, a large, clearly visible pyrenoid in the centre, and an oval nucleus in the lower part of the cell (Figs. 2b, 2c). The cell is covered with two large plates, which are joined by the sagittal suture (Fig. 2i). At the apical end of the left plate there is a small indentation (Fig. 2e), and at the right end there is a narrow V-shaped depression with a width of 5.56 µm and depth of 4.01 µm (Figs. 2d−2g). This is the periflagellar zone, where the flagellum and apical pores are located, as well as a number of small platelets (Figs. 2j−2l). The large thecal plates are smooth, with small depressions of 0.45−0.93 µm (0.64 ± 0.12 µm, n = 48) scattered over them with two types of pores: the predominant large ones of 0.12−0.21 µm (0.16 ± 0.03, n = 59) and small ones of 0.06−0.11 µm (0.09 ± 0.02, n = 13) in diameter (Fig. 2h). Depressions with pores are scattered almost all over the surface of large plates, are more densely located on the margins, and in most cases are absent in their central part. The number of pores on the plate is 203−310 (267 ± 34.9) and, at the marginal of zone, 134−164 (151 ± 9.2, n = 7). The Intercalary band is smooth (Fig. 2i). In the upper part of the periflagellar zone there are four small platelets; the second one is the smallest (Figs. 2j−2l).
Light (a−c) and electronic (d−l) microphotographs Prorocentrum leve. (a, b) view of different cells on the right side – (a) fixation with glutare and (b) fixation with Lugol solution; (c) view of the cell on the left side (fluorescent microscope); (d) view on the right side; (e) view on the left side; (f, g) lateral view of the cells; (h) pores; (i) view of the insertion belt; (j–l) periflagellar zone. (ap) is the apical pore, (fp) is the flagellar pore, (p) is the pirenoid, and (n) are the nucleus.
Prorocentrum leve algae occurrence in Lake Manych-Gudilo. In a study of phytoplankton in May 2016 (stations nos. 1−5) (Fig. 1) P. leve cells were not detected. In July, samples of plankton were taken in the northwestern part of the lake (stations nos. 6−8) at a heavy wave level; P. leve was present at these stations at a number of 1300−3826 cells/L (2−4% of the total phytoplankton number). Water temperature was 25.3−26.8°C and salinity was 44 g/L. Small green algae were dominated in the plankton (83−94% of total phytoplankton abundance). Dinoflagellates and diatoms accounted for 11 and 6%, respectively. In addition to P. leve, other marine species of dinoflagellates were found, such as Heterocapsa rotundata (Lohmann) Hansen and Akashiwo sanguinea (Hirasaka) Hansen et Moestrup.
In October, almost the entire water area of the lake was studied (stations nos. 6−18). However, P. leve, as in July, was recorded only on stations nos. 6−8 in the northwestern part of the lake. Numbers of the species reached 1111−1342 cells/L and were dominated by A. sanguinea. During this period, the water surface temperature reached 7−10°С and salinity was 38–44 g/L.
In February 2017, at 0.2−0.3°C and salinity 6–38 g/L, P. leve was absent in plankton and only a few fixed cells with compressed content were found on dead filamentous algae.
DISCUSSION
The morphology of P. leve from Lake Manych-Gudilo coincides with the first description of the species in terms of cell shape, absence of thecal ornamentation, and structure of the periflagellar zone [22]. According to M. Faust et al. [22], these features distinguish P. leve from other species of the genus Prorocentrum. Cells from the lake are larger than cells from the Caribbean and Aegean Seas, but fall within the size range specified for P. leve from the Bay of Biscay (Table 1). In contrast to cells from the sea coast, specimens from the lake had more pores on their large plates and in their marginal zone (Table 1). As in the cultures of P. leve from the Bay of Biscay, two types of pores were noted in the studied specimens on large plates: large pores and small ones. The diameter of the large pores coincided with that in the first description of the species (Table 1). In addition, the diameter of the depressions in which the pores were located was noticeably larger than that of the cells from the Bay of Biscay (Table 1). The morphological differences may be due to the process of the organism adapting to an unusual environment, which is characterized by a high degree of equally chloride and sulfate salinity.
Until now, P. leve has been observed exclusively in the zones of tropical [22], subtropical (northern Mediterranean Sea [18, 27, 29]), and temperate (Atlantic, off the coast of the Iberian Peninsula in the Bay of Biscay [19]) zons. This is the first record of P. leve in a continental waterbody, which is also characterized by high salinity. In addition to this species, representatives of other genera of marine dinoflagellates are registered in the lake, such as Akashiwo Hansen & Moestrup, 2000, Heterocapsa Stein, 1883, and Gyrodinium Kofoid & Swezy, 1921. Types of these genera were also found in the lake earlier under waterbody salinity ≤30 g/L [7, 16]. Under current conditions, at salt content ≥50 g/L, marine species Glenodinium pilula (Ostenfeld) Schiller, Scrippsiella acuminata (Ehrenberg) Kretschmann et al., Prorocentrum scutellum Schröder, Heterocapsa rotundata (Lohmann) Hansen were found among dinoflagellates [5, 6].
Dinoflagellate P. leve has not been previously found in Russian reservoirs. However, as early as in the 1930s, dinoflagellates of Еxuviaella caspica Kisselew were described by I.A. Kiselev [1] from the Northeastern Caspian bays with water salinity of 31−53‰. Later this species was found in the eastern part of the Sivash Bay of the Sea of Azov, water bodies of Ukraine, and lakes of flat Georgia [8]. In the mid-1970s, the genus Exuviaella Cienkowski, 1881, was abolished and the species was reduced to synonyms with Prorocentrum lima (Ehrenberg) Stein [20]. A.F. Krahmalny [3] considered this species to be an independent taxon and proposed a new nomenclature combination—Prorocentrum caspica (Kisselew) Krachmalny, but P. caspica was not included in the list of modern free-living dinoflagellates [23]. Actually, the shape and size of Exuviaella caspica coincide with those of P. leve. However, the wide oval shape and pyrenoid in the center of the cell are present not only in P. leve, but also in several types of benthic dinoflagellates, including P. lima, characterized by wide variability [25]. The description of the ornamentation of the E. caspica cell as “smooth or punctured” [1, p. 112] suggests that two morphologically similar species of benthic Prorocentrum inhabit the Caspian Sea. It is necessary to verify the species from the typical location described as E. caspica using modern research methods.
It’s hard to tell how and when P. leve entered Lake Manych-Gudilo. It is possible that this is an autochthonous species with a wide tolerance to salinity fluctuations, which the species successfully experienced in the form of cysts. In previous studies of plankton, P. leve was not noted [4–7, 10, 16], probably because it leads a predominantly epiphytic lifestyle [18, 22], and no studies of microalgae in the growths have been conducted here. The species was detected by us for the first time during a strong wave in the lake, as a result of which epiphyte organisms could be carried out in the water column. It remains unclear which biotope in this pond P. leve prefers, its seasonal dynamics and distribution in the lake, and whether the local population is able to produce a toxin.
CONCLUSIONS
Marine dinoflagellate P. leve was registered for the first time in an inland waterbody in Russia in hypersaline Lake Manych-Gudilo. Morphological characteristics of the species in general correspond to the first description, but there are a number of structural features. P. leve mass development in lake Manych-Gudilo was not achieved and was recorded only in the northwestern part of the reservoir in a vegetative state in July and October in plankton and in transition to cysts in February on algae mats. The finding of this species in Lake Manych-Gudilo may serve as indirect evidence of the fragmentation of seaweed habitats as a result of paleobasin regression and significantly expands knowledge about the composition of the algal flora of inland water bodies in Russia, as well as about the ecology and distribution of this marine species.
ACKNOWLEDGEMENTS
We are grateful to E.G. Alyoshina and the staff of the Southern Scientific Center of RAS for providing data on hydrochemistry; A.I. Savikin, V.V. Sayapin, and V.L. Semina for sampling; and M.V. Nabozhenko (Caspian Institute of Biological Resources of the Far East Branch, Russian Academy of Sciences) for valuable comments and advice during the preparation of this work.
FUNDING
The work was carried out as part of the state assignment of research work of the Southern Scientific Center of the Russian Academy of Sciences, the topic “Marine Biogeosystems of Southern Russia and Their Catchments in Arid Climate, Economic Development, and Modern Geopolitical Challenges” (state registration no. AAAAA-A18-118122790121-5), with partial support from a grant of the Russian Foundation for Basic Research (project no. 15-04-05331).
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
REFERENCES
Kiselev, I.A., Phytoplankton of the Northeast Caspian Sea with its bays Komsomolets (Mertvyi Kultuk) and Kaidak according to Materials of the expeditions of the Academy of Sciences of the USSR in 1934 and 1935, Tr. Kompleksn. Izuch. Kasp. Morya, 1940, no. 3, pp. 103–125.
Krakhmal’nyi, A.F., New taxonomic and nomenclatural combinations in Dinophyta, Algologiya, 1993, vol. 3, no. 4, pp. 87–88.
Krakhmal'nyi, A.F., Dinofitovye vodorosli Ukrainy (Dinoflagellates of the Ukraine), Kyiv: Alterpress, 2011.
Kruglova, V.M., Proletarskoe vodokhranilishche (Proletarskoe Reservoir), Rostov-on-Don: Rostov. Gos. Univ., 1972.
Luzhnyak, O.L., The finding of marine dinoflagellates in Lake Manych-Gudilo, in Mezhdunarodnaya konferentsiya “Estestvennye i invaziinye protsessy formirovaniya bioraznoobraziya vodnykh i nazemnykh ekosistem,” Tezisy dokladov (Natural and Invasive Processes of Formation of the Biodiversity of Aquatic and Terrestrial Ecosystems: International Conference, Abstracts of Papers), Rostov-on-Don, 2007, pp. 196–197.
Luzhnyak, O.L. and Glushchenko, G.Yu., Seasonal changes in phytoplankton of the water bodies of the Kuma–Manych Depression in 2010, in Mezhdunarodnaya konferentsiya “Aktual’nye problemy obespecheniya prodovol’stvennoi bezopasnosti yuga Rossii,” Tezisy dokladov (Current Problems of Food Safety in Southern Russia, Abstracts of Papers), Rostov-on-Don, 2011, pp. 73–75.
Manych-Chograi: istoriya i sovremennost' (Manych-Chograi: History and Modernity), Rostov-on-Don: Everest, 2005.
Matvienko, O.M. and Litvinenko, R.M., Identification Guide to Freshwater Algae of the Ukrainian SSR, vol. 3, part 2: Pyrrophyta, Kiev: Naukova dumka, 1977.
Matishov, D.G., Orlova, T.A., Gargopa, Yu.M., and Pavel’skaya, E.V., Long-term variability in the hydrochemical regime of the Manych–Chograi hydrological system, Water Resour., 2007, vol. 34, no. 5, pp. 527–531.
Mikhailovskaya, Z.N., Phytoplankton dynamics in saline Manych water bodies, in Nauchnaya konferentsiya posvyashchennaya 80-letiyu Rostovskogo universiteta, Tezisy dokladov (Scientific Conference Dedicated to the 80th Anniversary of the Rostov University, Abstracts of Papers), Rostov-on-Don, 1949, vol. 1, pp. 128–129.
Nabozhenko, M.V., Bulysheva, N.I., and Shokhin, I.V., Current state of macrozoobenthos in the water bodies of the Kuma–Manych Depression in summer, in Sovremennoe sostoyanie i tekhnologii monitoringa aridnykh i semiaridnykh ekosistem Yuga Rossii (The Current State and Technologies of Monitoring of Arid and Semiarid Ecosystems in Southern Russia), Rostov-on-Don: Yuzh. Nauchn. Tsentr Ross. Akad. Nauk, 2010, pp. 113–118.
Proshkina-Lavrenko, A.I. and Makarova, I.V., Vodorosli planktona Kaspiiskogo morya (Planktonic Algae of the Caspian Sea), Leningrad: Nauka, 1968.
Radchenko, I.G., Kapkov, V.I., and Fedorov, V.D., Prakticheskoe rukovodstvo po sboru i analizu prob morskogo fitoplanktona: uchebno-metodicheskoe posobie dlya studentov biologicheskikh spetsial’nostei universitetov (Practical Guidance on the Collection and Analysis of Marine Phytoplankton Samples: A Study Guide for Students of Biological Specialties of Universities), Moscow: Mordvintsev, 2010.
Rumyantsev, V.A., Drabkova, V.G., and Izmailova, A.V., Ozera evropeiskoi chasti Rossii (Lakes of the European Part of Russia), St. Petersburg: Lema, 2015.
Svitoch, A.A., Yanina, T.A., Novikova, N.G., et al., Pleistotsen Manycha (voprosy stroeniya i razvitiya) (Pleistocene of Manych (Structural and Developmental Issues)), Moscow: Geograf. fakul’tet Mosk. Goc. Univ., 2010.
Fushtei, T.V., Spring phytoplankton of Lake Manych-Gudilo, in Trudy zapovednika Rostovskii (Transactions of the Rostov Reserve), Rostov-on-Don: Severo-Kavkaz. Nauchn. Tsentr Vysshei Shkoly, 2002, vol. 2, pp. 81–88.
Khausman, K., Khyul’sman, N., and Radek, R., Protistologiya (Protistology), Moscow: Tov. Nauchn. Izd. KMK, 2010.
Aligazaki, K., Nikolaidis, G., Katikou, P., et al., Potentially toxic epiphytic Prorocentrum (Dinophyceae) species in Greek coastal waters, Harmful Algae, 2009, vol. 8, no. 2, pp. 299–311.
David, H., Laza-Martinez, A., Garcia-Etxebarria, K., and Riobó, P., Characterization of Prorocentrum elegans and Prorocentrum levis (Dinophyceae) from the southeastern Bay of Biscay by morphology and molecular phylogeny, J. Phycol., 2014, vol. 50, no. 4, pp. 718–726.
Dodge, J.D., The Prorocentrales (Dinophyceae). II. Revision of the taxonomy within the genus Prorocentrum, Bot. J. Linn. Soc., 1975, vol. 71, no. 2, pp. 103–125.
Ehrenberg, C.G., Dritter Beitrag zur Erkenntniss grosser Organisation in der Richtung des kleinsten Raumes, in Abhandlungen der Königlichen Akademie der Wissenschaften zu Berlin, Berlin: Druckerei der Koniglichen Akademie der Wissenschaften, 1835, pp. 145–336.
Faust M.A., Vandersea, M.W. Kibler, S.R., et al., Prorocentrum levis, a new benthic species (Dinophyceae) from a mangrove island, Twin Cays, Belize, J. Phycol., 2008, vol. 44, pp. 232–240.
Gómez, F., A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata), CICIMAR Oceánides, 2012, vol. 27, no. 1, pp. 65–140.
Hammer, U.T., Saline Lake Ecosystems of the World, Monographiae biologicae, Dordrecht: Junk Publ., 1986, vol. 59.
Hoppenrath, M., Chomerat, N., Horiguchi, T., et al., Taxonomy and phylogeny of the benthic Prorocentrum species (Dinophyceae)—a proposal and review, Harmful Algae, 2013, vol. 27, pp. 1–28.
Hoppenrath, M., Murray, S.A., Chomerat, N., and Horiguchi, T., Marine benthic dinoflagellates—unveiling their worldwide biodiversity, Kleine Senckenberg-Reihe, 2014, vol. 54, p. 276.
Ingarao, C., Lanciani1, G., Iorio, D.Di., et al., Prorocentrum levis and Coolia monotis in Abruzzo coastal waters (W. Adriatic), Harmful Algae News, 2010, no. 41, pp. 12–14.
Kameneva, P.A., Efimova, K.V., Rybin, V.G., and Orlova, T.Yu., Detection of dinophysistoxin-1 in clonal culture of marine dinoflagellate Prorocentrum foraminosum (Faust M.A., 1993) from the Sea of Japan, Toxins, 2015, vol. 7, no. 10, pp. 3947–3959.
Pistocchi, R., Guerrini, F., Pezzolesi, L., et al., Toxin levels and profiles in microalgae from the North-Western Adriatic Sea—15 years of studies on cultured species, Mar. Drugs, 2012, vol. 10, pp. 140–162.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by T. Borisova
Rights and permissions
About this article
Cite this article
Glushchenko, G.Y., Luzhniak, O.L., Dvadnenko, K.V. et al. First Recorded Marine Species Prorocentrum leve Faust, Vandersea, Kibler, Tester & Litaker (Dinophyceae) in an Inland Waterbody. Inland Water Biol 12, 133–139 (2019). https://doi.org/10.1134/S199508291902007X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199508291902007X