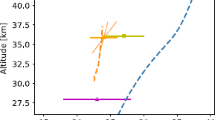Abstract
The chemical composition of the high-latitude of the middle atmosphere of the Northern Hemisphere, including the troposphere, stratosphere, and mesosphere, as well as its change in the 21st century, are considered. The initial data are obtained using the SOCRATES interactive radiation-chemical two-dimensional model, which makes it possible to calculate the altitude profiles of the components with a step of 1 km in the latitude zone 85° S–85° N in steps of 5°. The scenario of the Intergovernmental Panel on Climate Change (IPCC) RCP 4.5 for the conditions in June and January 2000 and 2100 and 50° N is used as the initial conditions. For these conditions, the height profiles and the total content of long-term components are obtained: N2O, CH4, and CO2; CFC-10 (CCl4), CFC-11 (CCl3F), CFC-12 (CCl2F2), CFC-113 (CCl2FCClF2), CFC-114 (CClF2CClF2), CFC-115 (CClF2CF3) chlorofluorocarbons; halon-2011 (CBrClF2) and halon-1301 (CBrF3); hydrochlorofluorocarbon HCFC-22 (CHClF2); HF, HCl, HBr, and HNO3 acids; and small atmospheric components: CH2O, O3,O(3P), O(1D), H, OH, HO2, H2O2, Cl, Cl2, ClO, OClO, HOCl, ClONO2, ClNO2, Cl2O2, N, NO, NO2, NO3, N2O5, HO2NO2, Br, BrO, HOBr, BrONO2, and BrCl, as well as the chemical families Ox (O3 +O(3P)), HOx (OH+HO2), NOx (NO + NO2 + NO3), ClOx (Cl + ClO), and BrOx (Br + BrO). It is shown, in particular, that for June 2100 compared to June 2000, the relative change in % in the total content of the components of the ClOx family in the stratosphere was –57.5%; in the Ox family, +4.0%; in the BrOx family, –25.7%; in the NOx amily, +13.9%, and in the HOx family, ‒4.1%. In January, the corresponding data for ClOx was –59.1%; for Ox, +7.3%; for BrOx, –26.2%; for NOx, +7.1; and for HOx, –3.6%. Similar comparisons are made for the other components mentioned above. Almost all chemically active components show a marked sensitivity to changes in season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 INTRODUCTION
The chemical composition of the atmosphere and its change over time are the most important characteristics of the environment, which make it possible to judge not only the current state of the latter in terms of its degree of safety for the environment and humans but also (with the reliability of forecasts) about future threats to the existence of people on Earth. The recent ozone crisis that took place at the end of the last century is a compelling example of this. It is known that as a result of the release of anthropogenic chlorofluorocarbons, the content of total ozone in the middle latitudes of the Northern Hemisphere at the end of the 20th century decreased by 4–6%, which amounted to a third of the difference between the amount of ozone today and 400 million years ago, when the ozone layer accumulated at that time sufficient thickness to allow life to emerge from the ocean, where it was protected from dangerous solar ultraviolet radiation by the water, to the surface of the Earth, under the protection of the ozone layer [1]. Projections of changes in the ozone layer have indicated that it could be further depleted to the levels present 400 million years ago. With a further uncontrolled increase in the production of chlorofluorocarbons, this may well happen, and then terrestrial life would again have to save itself in the ocean. Fortunately, a solution to the problem has been found. A remarkable feature of this discovery was that the solution was found theoretically in 1974, when there was no sign of the impact of chlorofluorocarbons on the ozone layer. They appeared later. It was a result of monitoring the atmospheric contents of ozone and chlorofluorocarbons, which changed in the opposite way, that it was finally proved that the proposed solution to save the ozone layer (and at the same time humanity) was correct and the only correct solution (meaning the decision to ban the production of ozone-depleting substances, which led to the adoption of international agreements in the form of the Montreal Protocol and its amendments). It is therefore not surprising that its authors (Molina, Rowland, and Crutzen) were awarded the Nobel Prize in 1995, 21 years after the discovery.
The modern problem of global warming is also directly related to the chemical composition of the atmosphere and requires continuous and careful monitoring of the atmospheric content of greenhouse gases and their changes. This is of particular importance for the Russian high-latitude zone due to the threat of the melting permafrost, which occupies 60% of the total area of the Russian Federation.
2 RESULTS AND DISCUSSION
2.1 Troposphere
The troposphere is adjacent to the Earth’s surface and located at heights of 0 to 10 km. Therefore, it is the troposphere that receives the natural and anthropogenic components emitted from the Earth’s surface. These include greenhouse gases—carbon dioxide (CO2), nitrous oxide (N2O), methane (CH4), and water vapor—as well as ozone (formed in the atmosphere itself); ozone-depleting chlorofluorocarbons, including carbon tetrachloride (CCl4), CFC-11 (CCl3F), CFC-12 (CCl2F2), CFC-113 (CCl2FCClF2), CFC-114 (CClF2CClF2), CFC-115 (CClF2CF3), halon-1211 (CBrClF2), halon-1301 (CBrF3), and their ozone friendly substitutes such as HFC-22 hydrochlorofluorocarbon (CHClF2); moreover, both of them have greenhouse properties. However, the troposphere serves not only as a reservoir for storing long-term components but various oxidative processes also take place in it. In relation to this , it should be pointed out that among the above compounds, the chemical composition of the troposphere is significantly affected by water vapor together with tropospheric ozone. This pair ensures the formation of the primary tropospheric oxidizer, the OH hydroxyl radical, according to the following scheme:
where \(J_{{{{{\text{O}}}_{{\text{3}}}}}}^{{\text{*}}}\) is the photodissociation coefficient, s–1; \({{k}_{{{\text{O}}{{{\text{(}}}^{1}}D{\text{)}} + {{{\text{H}}}_{{\text{2}}}}{\text{O}}}}}\) is the rate constant of the chemical reaction O(1D) + H2O, molecule–1 cm3 s–1. Reactions of OH radicals with CH4 and CO form an additional amount of ozone in the troposphere. The formation of ozone during the oxidation of methane occurs as a result of the following sequence of reactions involving nitric oxide NO:
It can be seen from scheme (3)–(9) that the process proceeds in a chain way, since the OH hydroxyl radical lost in reaction (3) is recovered in reaction (7). It should be pointed out that in a similar way (i.e., with the formation of ozone), methane oxidation proceeds at a sufficiently high concentration of NO, exceeding approximately 5–15 ppt. At lower NO concentrations, the process develops somewhat differently:
It can be seen that in this case, ozone is not formed, but only formaldehyde (CH2O) and water. Numerous so-called volatile organic compounds of natural (terpenes) and anthropogenic (carbon tetrachloride) origin are oxidized according to the same scheme as in the case of methane. A sufficient amount of NO is provided when the condition [NO] > (k10/k5)[HO2], where k10 and k5 are the rate constants of reactions (10) and (5), respectively; and [HO2] is the concentration of HO2 in cm–3.
Similarly, carbon monoxide CO is oxidized (also with the participation of NO):
Here, too, there is a chain process (reactions (14)–(9)) and a certain amount of NO is also required. However, unlike methane, with a lack of NO, ozone will not be formed, but destroyed, and at the same rate:
A sufficient amount of NO is provided when the condition [NO] > k17[O3]k7, where k17 and k7 are the rate constants of reactions (17) and (7), respectively, is fulfilled; [O3] is the concentration of O3 in cm–3.
In addition, OH radicals participate in reactions with all small H-containing components, including ozone-safe hydrochlorofluorocarbons, ensuring their relatively short lifetime and “ozone safety.”
Table 1 gives an idea of the change in the chemical composition of the troposphere in the 21st century, which shows the relative change in % in greenhouse and other trace atmospheric constituents in the troposphere, including CO2, N2O, CH4, H2O, CO, H2, O3, CFC-10 (CCl4), CFC-11 (CCl3F), CFC-12 (CCl2F2), CFC-113 (CCl2FCClF2), CFC-114 (CClF2CClF2), CFC-115 (CClF2CF3), hydrochlorofluorocarbon HCFC-22 (CHClF2), halon-1211 (CBrClF2), and halon-301 (CBrF3), in June 2100 compared to June 2000 (corresponding to the content of the corresponding component in 2000) at 70°N. All the data given in Table 1 are calculated using the SOCRATES 2D interactive model [2] and IPCC RCP 4.5 scenarios as the input data [3]. The magnitude of the effect was determined by the formula
where X2100 is the content in column 0–10 km of component X in June 2100 at 70° N, and X2000 is the same in June 2000 at the same latitude. It can be seen that the content of almost all components (with the exception of the main greenhouse gases, i.e., carbon dioxide and nitrous oxide) in 2100 significantly decreased compared to 2000, which is partly due to the regulation of their production under the Montreal Protocol and its amendments. We also note a significant increase in CO2 by 45.7% and in N2O, by 19.9%, which indirectly indicates the ongoing global warming in the 21st century, which is especially important for the northern regions due to the threat of the permafrost thawing and the subsequent economic, environmental, and other losses [4]. It can also be noted that the tropospheric ozone content in the 21st century has slightly increased (by about 2%), which is a consequence of competition, on the one hand, the negative trend of ozone precursors (CH4, 7%; CO, 6.41%), a negative effect, and on the other hand, the mainly negative trend of ozone depleters (CFCs, HCFCs, and halons, the content of which decreased by 5‒98%), a positive effect, although their role in ozone depletion is much less in the troposphere than in the stratosphere, whose changes in composition we now proceed to consider.
2.2 Stratosphere
From a chemical point of view, the stratosphere (located at heights of 10 to 55 km) is the main region of the atmosphere where the formation and destruction of atmospheric ozone occurs, which ultimately leads to a certain balance of all photochemical factors that determine the stationary (or close to it) state of the ozone layer of the Earth. We also point out that the stratosphere contains about 90% of all atmospheric ozone. It has now been established that the only source of ozone (not only in the stratosphere but in the entire atmosphere) is molecular oxygen, which, under the action of short-wave radiation, photodissociates into atoms with the further adherence of the formed O atoms to O2 molecules:
Complementing reactions (18) and (9) with the reaction
describing the loss of ozone in a pure oxygen atmosphere, we obtain the Chapman cycle [5], proposed in 1930. Although reactions (18), (9), and (19) do not form a chain process, it is reaction (19) that will determine the loss of ozone in all chain processes of ozone destruction discovered thirty years later.
These are the so-called catalytic cycles of ozone depletion, including cycles of Ox, HOx, NOx, ClOx, and BrOx, whose effect on the ozone layer is described in detail in the monograph [6]. The role of individual cycles and the history of their discovery are described in [7–20]. Of particular note is the work [13] (1958), which was the first to propose a catalytic cycle for the destruction of stratospheric ozone with the participation of OH radicals. The value of this discovery was that for the first time a schematic diagram of the catalytic cycle of ozone depletion was proposed. By substituting any other radical instead of OH into this scheme, any other catalytic ozone destruction cycle could be obtained. However, 13 years passed before Crutzen [8] had the idea to substitute NO instead of OH in this scheme and received the so-called nitrogen-oxide cycle, and at the same time was awarded the Nobel Prize. Other issues related to the chemistry of the stratosphere and the ozone layer are discussed in [9–15]. The works cited contain information that fully describes the processes taking place in the stratosphere and the factors that determine the current and future state of the Earth’s ozone layer. For this reason, we will not dwell on the details of the chain mechanism of stratospheric ozone destruction, but will present a general picture of the chemical composition of the stratosphere and its changes in the 21st century.
Figure 1 shows the height profiles of some stratospheric components, including O3,O(3P), H, OH, N, and NO for June 2000 and 70° N. It can be seen that O3 has the highest concentration, and in the upper stratosphere, also O(3P). They are followed by NO, OH, H, and N. We will return to this figure in the next section.
Figure 2 shows the relative (%) change in the concentrations of the Ox, HOx, NOx, ClOx, and BrOx families in June 2100 compared to June 2000 at 70° N. It can be seen that the odd oxygen family has “grown up” (especially in the upper stratosphere), which is explained by the significant drop in the content of the ozone-destroying chlorine and bromine families as a result of the restrictive measures of the Montreal Protocol and its amendments. (The concentration of the oxygen family increased by 30% in the upper stratosphere, while the concentration of the chlorine family fell by 50‒60% and the concentration of the bromine family by 30%.) The scale of changes in the concentration of the ClOx family during the 21st century and its difference from changes in the concentration of BrOx are due to the differences in the application of the Montreal Protocol and its annexes to chlorine- and bromine-containing anthropogenic ozone depleters.
The effects described above are mainly due to external factors, which refers to measures such as the implementation of the Montreal Protocol. However, in addition to these factors, the contents of the family components also change under the influence of internal factors, such as changes in atmospheric conditions during the change of season. Figure 3 shows the relative change in % in the concentrations of families during the transition from January to June in 2000 at 70° N. The effect shown in Fig. 3 was calculated by the formula
where XJan is the height profile of the concentration of family X in January 2000 at 70° N; and XJune, in June 2000 at the same latitude. It can be seen from formula (II) that the negative effect cannot be less than 100%, and the positive effect can be arbitrarily large.
It can be seen that the change in the season leads to significant changes in the concentrations of families, exceeding the changes over 100 years. For example, the concentration of the Ox family in the upper stratosphere changes by more than 50%; and that of NOx, by 250%. Seasonal changes in the concentrations of the HOx, ClOx, and BrOx families are close to the maximum, i.e., to –100%. These changes are related both to changes in the conditions of the occurrence of photochemical and chemical reactions (lack of sunlight during the polar night and lower temperatures in January compared to June) and to changes in the transport conditions. As for the HOx, ClOx, and BrOx families, the closeness of the change in their concentrations to –100% is explained by the fact that, under polar night conditions, the active components of these families, which initially have a “summer” concentration, quickly disappear in the recombination reactions or in reactions with other stratospheric components, as a result of which XJan becomes smaller than XJune, which leads to the maximum negative effect. The positive and rather high seasonal changes in the concentration of the Ox family are explained solely by the effect of dynamic factors, since in their absence they would be zero. The latter is explained by the fact that ozone does not die at night: without O(3P) atoms, which quickly die at night in the O(3P) + O2 → O3 reaction, the ozone’s molecules can react with each other, but the O3 + O3 → 3O2 reaction is too slow, and it provides a virtually infinite ozone lifetime. Therefore, ozone brought to high latitudes by global circulation processes will be conserved and accumulate here in January. This will lead to the fact that its content in January will become larger than in June, which is confirmed by the results of calculations. Similarly, the seasonal variation in the concentrations of the NOx family without taking into account dynamic processes, would also be zero, since with any changes in the ozone content, the O3 + NO → NO2 +O2 reaction can only change the ratio between the NO and NO2 concentrations, but not their sum. This is directly evident from the data presented in Fig. 3, where the curves for Ox and NOx behave differently without any relation to each other. The scale of changes in the concentration of the ClOx family during the 21st century and their difference from changes in the concentration of BrOx are due to the differences in the application of the Montreal Protocol and its annexes to chlorine- and bromine-containing anthropogenic ozone depleters.
In general, the change in the chemical composition of the stratosphere in the 21st century is mainly due to the cessation of the production of anthropogenic ozone-depleting substances and the subsequent natural process of their removal from the atmosphere, the rate of which is determined by the atmospheric lifetime of a particular compound. The combination of these processes determines the recovery time of the Earth’s ozone layer.
2.3 Mesosphere
The mesosphere, located at heights of 56 to 85 km, belongs to the middle atmosphere in which its constituent gases are mixed. This is explained by the fact that the altitude distribution of gases is determined by the processes of turbulent diffusion, and not by gravity, as in the thermosphere and at higher altitudes. If no other factors (except diffusion) “worked,” then the altitude distribution of any atmospheric components, expressed as the ratio of the concentration of this component at the given height to the concentration of the main gases of the atmosphere (N2 and O2) at the same height, i.e., in volume mixing ratio units and in volume mixing ratio coordinates, the height would look like vertical lines at all heights. In real conditions, atmospheric components are involved in chemical and photochemical processes, which distorts the picture. An example of the altitude distribution of some long-term and short-term atmospheric components in the stratosphere and mesosphere for the conditions of June 2000 and 70° N are shown in Fig. 4. It can be seen that only components that live more than 1000 years have a vertical distribution, while components with lifetimes of the order of 100 years reach the mesosphere with large losses, and those that live less than 100 years may not reach the mesosphere at all. We also note that the deviation of the height distribution of the component to the right from the vertical means the presence of the process of its formation in the atmosphere, and the deviation to the left means the presence of the destruction process.
Bearing in mind that there are very few anthropogenic substances with atmospheric lifetimes of more than 1000 years, it can be concluded that the mesosphere is an ecologically clean zone, practically not polluted by anthropogenic and natural emissions from the Earth’s surface. Therefore, the chemical composition of the mesosphere is largely determined by the natural components of the atmosphere and the short-wave UV radiation of the Sun, which changes little with time and is unlikely to change in any way over the next 100 years.
As an example of the modern chemical composition of the mesosphere, Fig. 5 shows the concentration profiles of some minor components of the mesosphere for June 2000 and 70° N. Comparing these data with the data presented in Fig. 1, it can be seen that the ozone concentration in the mesosphere continues to decrease with increasing altitude, which is mainly due to the decrease in the rate of triple collisions, which result in the formation of ozone (see reaction (9)). At the same time, the concentration of O atoms, which in the stratosphere was noticeably lower than that of ozone molecules, increases in the mesosphere and, at altitudes above 60 km, begins to exceed the concentration of O3, which is also explained by the decrease in the rate of triple collisions in which oxygen atoms die. For the same reason, the concentrations of H and N atoms increase, and the concentration of H at heights above 65 km begins to exceed the concentration of OH radicals.
In general, it can be stated that the chemical composition of the mesosphere is largely determined by natural factors and, above all, by the level of short-wave solar radiation, as a result of which it (the composition) undergoes relatively weak changes with time. This, in particular, is confirmed by the data shown in Fig. 6, which shows the relative change in the altitude profiles of the concentrations of the Ox, HOx, NOx, ClOx, and BrOx families in June 2100 compared to June 2000. Comparison of these data with the data shown in Fig. 2 for the stratosphere shows that changes in the concentrations of families over 100 years in the mesosphere are much smaller than in the stratosphere. Causes of the differences in the changes in the concentrations of the ClOx and BrOx families in the mesosphere are the same as in the stratosphere, except for the fact that the content of halons in the mesosphere is very low, which possibly reduces the mesospheric effect in the bromine family compared to the stratospheric effect.
3 CONCLUSIONS
1. The chemical composition of the high-latitude of the middle atmosphere, including the troposphere, stratosphere, and mesosphere, as well as its change in the 21st century, is considered.
2. It is shown that the main reason for the changes in the chemical composition of the troposphere and stratosphere in the 21st century are the measures taken to protect the ozone layer (the Montreal Protocol and its amendments).
3. It has also been shown that the components of chemical families are extremely sensitive to changes in season, and the changes in their concentrations during the transition from winter to summer significantly exceed the changes that occur over the course of a century.
4. It is shown that the chemical composition of the mesosphere and its change are determined mainly by natural factors.
REFERENCES
R. Wayne, Chemistry of Atmospheres, 2nd ed. (Clarendon, Oxford, 1991).
http://acd.ucar.edu/models/SOCRATES/.
http://tntcat.iiasa.ac.at:8787/RcpDb/dsd?Action=htmlpage&page=welcome.
O. A. Anisimov and M. A. Belolutskaya, Meteorol. Gidrol., No. 6, 15 (2002).
S. Chapman, Met. R. Met. Soc. 3, 103 (1930).
I. K. Larin, Chemical Physics of Ozone Layer (RAN, Moscow, 2018) [in Russian].
B. G. Hunt, J. Atmos. Sci. 23, 8 (1966).
P. J. Crutzen, J. Geophys. Res. 76, 7311 (1971).
H. S. Johnston, Science (Washington, DC, U. S.) 173, 517 (1971).
R. S. Stolarski and R. J. Cicerone, Can. J. Chem. 52, 1610 (1974).
S. C. Wofsy and M. B. McElroy, Can. J. Chem. 52, 1582 (1974).
M. J. Molina and F. S. Rowland, Nature (London, U.K.) 249, 810 (1974).
W. D. McGrath and R. G. W. Norish, Nature (London, U.K.) 182, 235 (1958).
D. J. Jacob, Introduction to Atmospheric Chemistry (Princeton Univ. Press, Princeton, 1999).
G. P. Brasseur and S. Solomon, Aeronomy of the Middle Atmosphere (Springer, Dordrecht, 2005).
I. K. Larin, Russ. J. Phys. Chem. B 14, 344 (2020).
I. K. Larin, Russ. J. Phys. Chem. B 13, 867 (2019).
I. K. Larin, Russ. J. Phys. Chem. B 13, 548 (2019).
I. K. Larin, A. E. Aloyan, and A. N. Yermakov, Russ. J. Phys. Chem. B 15, 577 (2021).
I. K. Larin, A. E. Aloyan, and A. N. Yermakov, Russ. J. Phys. Chem. B 15, 357 (2021).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larin, I.K. Chemical Composition of the High Latitude of the Middle Atmosphere of the Northern Hemisphere and its Changes in the 21st Century. Russ. J. Phys. Chem. B 16, 492–498 (2022). https://doi.org/10.1134/S1990793122030083
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793122030083