Abstract
The results of studies on the extraction of vanadium, nickel, and cobalt compounds from ash residue obtained in the process of the filtration combustion of charcoal and brown coal with subsequent hydrometallurgical extraction of metals from ash residues are presented. Coals with metal salts preliminarily deposited on them are used for the research. The regularities of gasification of the studied coals (temperatures and combustion rates) are studied and it is shown that the heat of combustion of gaseous products for both types of coals is 4.3–4.5 MJ/m3. Using X-ray phase analysis, the crystal structures of metal compounds in ash residues are determined. Metal compounds are leached from the ash residue with water, acids, hydrogen peroxide, and their mixtures. It is established that vanadium compounds almost completely turn into a solution during leaching with acid solutions. At the same time, the maximum degree of extraction of nickel and cobalt from the ash residues of gasification does not exceed, respectively, 59 and 61% for charcoal and 40 and 28% for brown coal. This is due to the fact that nickel and cobalt compounds are present in ash residues, which are resistant to the action of aqueous solutions of acids and other oxidizing agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Due to their specific physical and chemical properties, rare earth elements are widely used in many high-tech applications (energy, electronics, space, etc.) [1]. The main difficulty in obtaining rare earth elements is the lack of their concentrated deposits. The main sources of obtaining rare earth elements include mineral ores [2], used electronics [3], etc. Currently, increasing attention is paid to coals and oils, the ash of which also contains many rare earth elements, especially metals [4]. Various methods are being developed for the selective isolation of rare earth elements: extraction [5], sorption [6, 7], metallurgical [8, 9], biological [10, 11], etc.
Coals and petroleum coke are widely used in the power industry. Gasification is an efficient method of obtaining energy from solid fuels. Gasification of fuels can be carried out both in autothermal [12–14] and allothermal modes [15, 16]. Previously, the characteristics of combustion and mass transfer of metal-containing products in the mode of filtration combustion were studied in [17, 18]. During the gasification of solid fuels in the mode of filtration combustion, the so-called superadiabatic effect is observed due to heat exchange between the reactants and products [19, 20]. The advantages of the superadiabatic method of gasification of solid fuels in comparison with the known technical solutions consist of the energy efficiency of the process, which makes it possible to process low-calorie mixtures and low content of toxic substances in gaseous combustion products [21–23].
To extract V, Ni, and Co from the ash residue, a number of technological processes have been proposed, which can be divided into pyrometallurgical and hydrometallurgical ones. Pyrometallurgical methods of ash processing include chlorination or reduction smelting to obtain alloys of V compounds and other metals [24, 25]. To carry out the chlorination of ash products collected after the gasification of coals or other types of solid fuels, carbon must be present in their composition. In industrial samples of ash products, carbon particles are always contained in the form of undercombustion. It is indicated [26] that ash and slag waste (ASW) granulation is initially carried out. Granules are recommended to be granulated at temperatures of 500 to 700°C in the molten salts of KCl and NaCl. For example, chlorination of V compounds (600–700°C) is described by the equation
After cooling the chlorination products, either fractional condensation of individual chlorine-containing compounds V, Ni, and Co, which differ in condensation temperatures, can be carried out, or their mixtures can be obtained, from which pure metal compounds are isolated by rectification. The process of chlorination of various types of raw materials is used on an industrial scale in many countries, including Russia.
Another method for processing ash products is the smelting of alloys of the considered metals with iron compounds to obtain ferroalloys containing vanadium, ferronickel, and cobalt [27, 28]. The most promising methods are hydrometallurgical processing, which allow the use of ash with a low content of compounds of the considered metals. The extraction of V, Ni, and Co from coal ash by hydrometallurgical methods can be carried out by acid [29], alkaline, or water leaching [30–33]. The hydrometallurgical extraction method was used in Russia on an industrial scale at the Tulachermet plant to obtain V2O5 from the ASW collected after the combustion of fuel oil. The most complete hydrometallurgical methods are studied in the works of V.I. Bukina et al. [34].
The patent [35] proposes a method for processing the ASW obtained after the combustion of fuel oils, which consists in mixing them with sodium carbonate and water. Next, the resulting mixture is kept at a constant temperature in the range of 100 to 150°C (in a furnace) for 2 hours to obtain a sinter. From the dried self-disintegrating cake, the degree of extraction of V by water leaching at a temperature of 95 to 100°C is 73–74%.
The method for leaching V and Ni from the ash residue with hydrochloric acid was patented in the United States [36]. According to this method, the ash residue was mixed with hydrochloric acid, the suspension was filtered, and the filtrate was treated with sodium, potassium, or calcium hydroxide, increasing the pH of the solution to 5.5–6.5. In this case, a precipitate containing V compounds was formed, which was filtered off and then additionally leached at pH 8.5–9.5, precipitating nickel hydroxide. The precipitate containing vanadium compounds was dried, mixed with sodium, potassium, or calcium hydroxide, and then the mixture was calcined in air at 500–1000°C. The result was a solid product containing pentavalent vanadium, which was leached with water and filtered. The filtrate was acidified with hydrochloric acid and precipitated with V2O5.
In [37], an electrochemical method was proposed for leaching a vanadium compound from an ash residue using aqueous solutions of alkali metal chlorides and carbonates as an electrolyte. The method does not seem acceptable, since electrolytes containing chloride ions cause equipment to corrode, and the use of carbonates is related to the difficulty of regenerating the spent electrolyte.
The choice of a reagent and the conditions for carrying out the hydrometallurgical processing of ash residues in order to extract metal compounds depend on the material composition of the residues, the conditions for gasification, and the elemental composition of the initial coal. The thermodynamic evaluation of the distribution between the phases of the compounds of the considered metals (cobalt, nickel, vanadium) showed that these metals under the conditions of the filtration combustion wave should remain in the solid phase in the form of oxide forms.
The aim of this paper is to study the possibility of increasing the degree of the useful use of solid fuel (coal) through the complex processing of resources and heat generation during coal gasification, followed by the release of rare metals from the ash residue.
EXPERIMENTAL
Analytical Research Methods
Elemental analysis of the organic mass of coal was carried out by combustion in an oxygen flow on a Vario MICRO cube elemental CHNS/O analyzer manufactured by Elementar Analysensystem GmbH (Germany) with a dynamic range of element registration from 0.01 to 100%. The moisture content in coals was determined by GOST (State Standard) R 52911-2013 “Solid mineral fuel. Determination of total moisture”; and the ash content, according to GOST R 55661-2013 “Solid mineral fuel. Determination of ash content.” The gaseous products of the filtration combustion of coals were analyzed on the gas chromatograph Khromatek Kristall-5000.2 manufactured by ZAO SKB Khromatek (Russia).
The contents of V, Ni, and Co compounds in the model coal samples, ash residues after ash leaching, and filtrates were determined on an X-ray fluorescent ARL PERFORM’X Sequential XRF spectrometer produced by TERMO FISHER SCIENTIFIC (United States) and an emission spectrometer with inductively coupled plasma ICPE-9000 manufactured by SHIMADZU (Japan). The phase composition of ash residues was studied on a Rotaflex RU-200 X-ray diffractometer manufactured by Rigaku (Japan), operating according to the following source parameters: 50 kV, 160 mA. The wavelength of monochromatized radiation was 1.542 Å. For monochromatization, a graphite crystal-monochromator was used on a beam reflected from the sample. The source was equipped with a Rigaku D/Max RC horizontal goniometer; θ–2θ scanning was performed according to the Bragg–Brentano scheme in the angular range 2θ = 10°–70°. The measurement was carried out in the continuous scanning mode at a rate of 2°/min and a step of 0.04°. The experimental diffraction patterns were processed using the specialized MDI Jade 6.5 program. The phases were identified using the international electronic database of diffraction data ICDD PDF-2.
Method for Preparing Coal Samples
Charcoal (GOST 7657-84) and sulfurous coal were chosen as the model coal samples. Moscow Region Basin of the Kimovsky open-pit mine (brown coal). The particle size of charcoal was 3–5 mm; that of brown coal, 3–7 mm. The initial coal was dried to an absolutely dry state, after which it was weighed on a laboratory VST-600/10 electronic balance.
Coals were impregnated with the following salts of cobalt, nickel, and vanadium:
(1) cobalt(II) nitrate hexahydrate (Co(NO3)2∙6H2O, GOST 4528-78, purity not less than 99%).
(2) nickel(II) nitrate hexahydrate (Ni(NO3)2∙6H2O, GOST 4055-78, purity not less than 98%).
(3) ammonium metavanadate (NH4VO3, GOST 9336-75, purity not less than 99%).
The amount of addition of metal salts was calculated so that the metal content in coal was 0.1 wt %. Metal salts were weighed on an analytical balance with the smallest weighing limit of 0.0001 g.
Metal salts were dissolved in distilled water, and the volume of water was chosen so that the coal was completely immersed in the solution. Salts of cobalt and nickel salts, which are easily dissolvable in water, were dissolved in cold water; and ammonium metavanadate, in hot water.
Dry coal was poured into a wide cuvette and filled with the prepared solution. After part of the solution was absorbed by the charcoal, the remainder of the solution was poured into a glass beaker, and the wet charcoal was placed in an oven. The dried coal was reimpregnated with the rest of the solution, mixed and dried again; and this procedure was repeated until the entire solution was completely absorbed. The final stages involved washing off the dissolved substance deposited during evaporation of the solution on the walls of the cuvette with a small amount of water, impregnating the coal with it, and completely drying the coal in an oven at 105°C. Before combustion, a representative sample was taken from the total mass of impregnated coal, which was ground in a mortar and analyzed for its metal content.
Bottom Ash Leaching Method
Before carrying out analyses and experimentally studying the possibility of extracting V, Ni, and Co compounds from coal ash into a solution, the ash products were subjected to mechanical grinding to an analytical fineness in a porcelain mortar. Leaching was carried out in a 300-mL flat-bottomed flask placed on a heated magnetic stirrer. A weighed portion of ash weighing 2 g was loaded into a flask, then a leaching reagent was added at different mass ratios of ash (T) and leaching solution (L) ranging from 1/10 to 1/30. The treatment was carried out at a temperature of 60°C for 2 hours. Next, the resulting suspension was filtered on a Buchner funnel. The filter with the filtered material was washed with water, dried, weighed, and the mass was determined for balance. Next, a sample was taken from the solid residue in the filter and the content of V, Ni, and Co was determined, and the degree of their extraction was determined from the obtained data on their contents in the initial ash and the filter residue.
The following substances were used as leaching reagents:
– distilled H2O;
– 25% NH4OH (GOST 3760-79) + 5% (NH4)2CO3 and 25% NH4OH + 10% (NH4)2CO3;
– H2O2 with a concentration of 37% (GOST 177-88);
– H2SO4 with a concentration of 95% (GOST 4204-77) and 20%;
– HNO3 with a concentration of 65% (GOST 4461-77);
– a mixed solution containing 650 g/L H2SO4 and 250 g/L HNO3;
– HCl with a concentration of 35 to 38% (GOST 3118-77);
– NaOH solution with a concentration of 20%.
EXPERIMENTAL
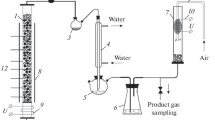
Experimental studies on the filtration combustion of metal-containing coals were carried out on a batch laboratory installation, the scheme of which is shown in Fig. 1. The main part of the experimental setup is a vertically located cylindrical reactor 3 made of quartz glass with an inner diameter of 66 mm, wall thickness of 3 mm, and ength of 500 mm. To reduce lateral radiation heat losses, the outer wall of the reactor was shielded with a heat-reflecting screen, 4.
The investigated samples of coals were loaded into the reactor through the upper end. Air was supplied through a fitting at the lower end of the reactor. The air flow was controlled using an electronic Mass-view flow meter, 5 (Fig. 1) manufactured by Bronkhorst High-Tech (Netherlands). The product gas was removed through the upper end of the reactor.
Before the start of the experiment, a layer of inert material was loaded into the reactor up to the level of the first thermocouple (TC1). Ceramic corundum balls with a diameter of 5 to 7 mm were used as the inert material. An initiating composition was poured over a layer of ceramic balls, which was a mixture of charcoal (10 g) with an inert material (30 g) heated in a muffle furnace to ~800°C. After that, the supply of the oxidizer (air) to the reactor was started. At the same time, the combustion of the initiating composition began. The air flow rate in all experiments was maintained constant at 800 m3/m2 h (0.76 L/s).
Next, the test mixture was loaded in small portions (~50 g). After 5–7 min, a stationary combustion front was formed, which was characterized by a constant combustion rate and temperature. The propagation of the filtration combustion wave front in the reactor was recorded from the readings of six chromel–alumel thermocouples (TC1–TC6) located along the length of the reactor at a distance of 65 mm from each other. The thermocouple junction was located in the center of the reactor. Thermocouple readings were transmitted via ADC 2 to recording computer 1 (Fig. 1) and displayed on the monitor screen in real time.
The mass combustion rate was determined from the advancement of the combustion front recorded with the help of thermocouples and from the loss of the fuel mass during the experiment. In all experiments, gaseous products were periodically sampled in a glass ampule-trap. After the experiment, the solid combustion products were unloaded from the reactor, and the ash was separated, ground in a mortar, and analyzed according to the method described above.
RESULTS AND DISCUSSION
The elemental composition of the initial coals is given in Table 1. Unlike charcoal, brown coal is characterized by a high ash content and low calorific value.
The analysis of the metal content in the prepared samples of coals and their ash is presented in Table 2. Ash residues from the gasification of brown and charcoal impregnated with salts of vanadium, nickel, and cobalt can be used to study the processes of their extraction. The content of all three metals individually in the ash of charcoal is higher than their content in the ash of brown coal due to a significant difference in the ash content of coals. In a number of cases, a reduced content of metals in coal ash was observed due to their low concentration in the initial samples and losses during the unloading of the ash residue from the reactor.
In addition to the metals introduced by us, the impregnated coals also contained some chemical elements that were contained in the original coals (Table 3). These elements, according to the X-ray phase analysis, do not form crystalline phases with the considered metals.
During the air gasification of charcoal, the combustion temperature was ~1200°C, the mass combustion rate was 0.14 g/s, and the calorific value of gaseous products was 4.5 MJ/m3 (Table 4). In the case of brown coal, the temperature and combustion rate were higher than for charcoal. This was due to the higher ash content, resulting in a transitional structure of the combustion wave, characterized by higher temperatures and combustion rates. However, the heat of combustion of gaseous products is somewhat lower than that of charcoal and is ~4.3 MJ/m3. However, unlike charcoal, the products of the gasification of brown coal, in addition to gaseous products, contain combustible organic products of thermal decomposition (pyrolysis) of coal–tar (elemental composition, wt %: C, 74.8; H, 7.6; O, 13.0; N, 1.1; S, 3.5). The yield of pyrolysis products is largely determined by the temperature mode of the process [38, 39]. In our experiments, the yield of pyrolysis resins was ~8% of the initial mass of brown coal. The heat of combustion of the latter is ~32 MJ/kg.
According to the data of the X-ray phase analysis of wood (Fig. 2) and brown (Fig. 3) coal ash impregnated with V, Ni, and Co salts, the samples contain both individual oxide forms of these metals and their complex compounds. In a sample of charcoal ash impregnated with V, Ni, and Co salts, the main identified phases are VO2, NiO, NiTiO3, CoO, CaO, Ca2SiO4, CaSO4, and SiO2, while in in a sample of brown coal ash impregnated with the same salts, such phases are VO2, NiO, NiS2, NiCo2O4, Co3O4, Co2C, CaCO3, TiO2, Al6Si2O13, and Fe2O3.
Ash residues obtained after gasification of coals impregnated with V, Ni, and Co compounds are characterized by a high content of these metals, and the material composition (Figs. 2, 3) shows that the samples contain hard-to-recover compounds of some of the considered metals (NiTiO3, NiS2, NiCo2O4, Co2C). The resulting ash residues from the gasification of charcoal and brown coal, impregnated with V, Ni, and Co salts were heterogeneous products. Photographs of brown coal ash before and after grinding are shown in Figs. 4 and 5.
The selected reagents have different effects on the degree of extraction of vanadium, nickel, and cobalt compounds (Table 5). For example, under the action of a 20% NaOH solution, distilled water, and a mixture of NH4OH and (NH4)2CO3 on brown coal ash, the degree of recovery of V, Ni, and Co do not exceed 54, 34, and 22%, respectively. Under the influence of strong acids (H2SO4, HNO3, HCl and their mixtures), at the first stage of processing, the degree of extraction of V increases to 73–76%, while the extraction of Ni decreases to 29–30%, and the degree of extraction of Co compounds practically does not change. When carrying out additional stages of leaching (two or three stages), almost the complete extraction of vanadium compounds into the solution is achieved, and the degree of extraction of nickel and cobalt compounds also increases. The highest value of the degree of extraction of nickel (35%) and cobalt (25.5%) compounds is achieved with two-stage leaching when HCl and mixtures of HCl with H2SO4 and HNO3 are used as the reagent.
The effect of the composition of solutions on the degree of extraction of metals during the leaching of charcoal ash is consistent with the results obtained in the leaching of brown coal ash; however, in all the experiments, the degree of extraction of Ni and Co compounds is significantly higher, which is apparently due to the difference in the material compositions of the obtained ash residues. Compounds of some elements (Na, Ca, K, etc.) contained in the feedstock also pass into the resulting solution after four-stage processing.
Apparently, the low degree of extraction of Ni and Co from the studied samples of ash residues is related to the formation of compounds during gasification (NiTiO3, NiS2, NiCo2O4, Co2C), without the additional thermochemical treatment of which (sintering, chlorination, etc.) it is practically impossible to increase the degree of extraction of compounds of these metals.
CONCLUSIONS
The performed studies have shown that the rate and temperature of the filtration combustion of brown coal from the Moscow Region basin is higher than that of charcoal. As a result of the filtration combustion of both types of coals, a gas with a calorific value of 4.3 to 4.5 MJ/m3 is obtained. During the filtration combustion of brown coal, tar is also formed with a calorific value of ≈32 MJ/kg, the output of which is approximately 8% of the mass of the original coal.
The crystal structures of metal compounds in ash residues of coals have been determined. Experiments have been carried out on the leaching of metal compounds with water, acids, and hydrogen peroxide and their mixtures. It has been established that vanadium compounds almost completely (97–98%) turn into a solution during two-three-stage leaching with acid solutions. The ash residues contain nickel and cobalt compounds that are resistant to the action of aqueous solutions of acids and oxidizers. As a result, the maximum recovery of nickel and cobalt from ash residues did not exceed 59–61%.
REFERENCES
A. R. Chakhmouradian and F. Wall, Elements 8, 333 (2012). https://doi.org/10.2113/gselements.8.5.333
J. Demol, E. Ho, K. Soldenhoff, et al., Hydrometallurgy 188, 123 (2019). https://doi.org/10.1016/j.hydromet.2019.05.015
S. M. Jowitt, T. T. Werner, Z. Weng, et al., Curr. Opin. Green Sustain. Chem. 13, 1 (2018). https://doi.org/10.1016/j.cogsc.2018.02.008
E. A. Salgansky, M. V. Tsvetkov, Kh. M. Kadiev, M. Ya. Visaliev, and L. A. Zekel’, Russ. J. Appl. Chem. 92, 1616 (2019). https://doi.org/10.1134/S1070427219120024
Yu. I. Isaeva, A. M. Elokhov, S. A. Denisova, and O. S. Kudryashova, Russ. J. Phys. Chem. A 94, 1346 (2020)
V. F. Gromov, M. I. Ikim, G. N. Gerasimov, and L. I. Trakhtenberg, Russ. J. Phys. Chem. B 15, 140 (2021). https://doi.org/10.1134/S1990793121010036
M. Touré, J. Chamieh, G. Arrachart, et al., Sep. Purif. Technol. 251, 117330 (2020). https://doi.org/10.1016/j.seppur.2020.117330
Q. Tan, J. Li, and X. Zeng, Crit. Rev. Environ. Sci. Technol. 45, 749 (2015). https://doi.org/10.1080/10643389.2014.900240
Y. Lu and Z. Xu, Resour. Conserv. Recycl. 113, 28 (2016). https://doi.org/10.1016/j.resconrec.2016.05.007
T. Hennebel, N. Boon, S. Maes, et al., New Biotechnol. 32, 121 (2015). https://doi.org/10.1016/j.nbt.2013.08.004
J. C. Lee and B. D. Pandey, Waste Manag. 32, 3 (2012). https://doi.org/10.1016/j.wasman.2011.08.010
N. Ripoll, E. Salgansky, and M. Toledo, Int. J. Heat Mass Transfer. 177, 121472 (2021). https://doi.org/10.1016/j.ijheatmasstransfer.2021.121472
B. S. Seplyarskii, N. I. Abzalov, R. A. Kochetkov, and T. G. Lisina, Russ. J. Phys. Chem. B 15, 242 (2021). https://doi.org/10.1134/S199079312102010X
M. Fierro, P. Requena, E. Salgansky, et al., Chem. Eng. J. 425, 130178 (2021). https://doi.org/10.1016/j.cej.2021.130178
D. V. Antonov, T. R. Valiullin, R. I. Iegorov, et al., Energy 119, 1152 (2017). https://doi.org/10.1016/j.energy.2016.11.074
Ya. Solomatin, N. E. Shlegel, and P. A. Strizhak, Fuel 255, 115751 (2019). https://doi.org/10.1016/j.fuel.2019.115751
G. E. Zaslavskii, D. B. Lempert, and G. B. Manelis, Khim. Fiz. 33 (1), 14 (2014). https://doi.org/10.7868/S0207401X14010142
N. A. Lutsenko and E. A. Salgansky, Int. J. Multiphas. Flow 140, 103670 (2021). https://doi.org/10.1016/j.ijmultiphaseflow.2021.103670
N. Evseev, M. Ziatdinov, V. Romandin, et al., Processes 8, 1056 (2020). https://doi.org/10.3390/pr8091056
E. A. Salgansky, A. Y. Zaichenko, D. N. Podlesniy, et al., Int. J. Hydrogen Energy 45, 17270 (2020). https://doi.org/10.1016/j.ijhydene.2020.04.177
S. V. Kostin, P. M. Krishenik, and S. A. Rogachev, Russ. J. Phys. Chem. B 15, 68 (2021). https://doi.org/10.1134/S1990793121010073
N. A. Lutsenko, Combust. Theory Modell. 22, 359 (2018). https://doi.org/10.1080/13647830.2017.1406617
D. Podlesniy, A. Zaichenko, M. Tsvetkov, et al., Fuel 298, 120862 (2021). https://doi.org/10.1016/j.fuel.2021.120862
M. Ya. Shpirt and V. V. Rashevskii, Microelements of Fossil Fuels (Kuchkovo Pole, Moscow, 2010) [in Russian].
T. P. Sirina, T. I. Krasnenko, G. V. Solov’ev, et al., Vestn. YuUrGU, Khim. 5 (1), 4 (2013).
S. N. Khadzhiev and M. Ya. Shpirt, Microelements in Oils and Products of their Processing (Nauka, Moscow, 2012) [in Russian].
A. S. Shapovalov, A. V. Polishchuk, D. P. Chernykh, et al., RF Patent No. 2677197, Byull. Izobret., Polez. Modeli, No. 2 (2019).
A. A. Golubev and Yu. A. Gudim, RF Patent No. 2336355, Byull. Izobret. No. 29 (2008).
Kh. M. Kadiev, M. Ya. Visaliev, L. A. Zekel’, and M. Ya. Shpirt, Solid Fuel Chem. 52, 392 (2018). https://doi.org/10.3103/S0361521918060058
M. Ya. Visaliev, Cand. Sci. (Chem.) Dissertation (Topchiev Inst. Petrochem. Synth. RAS, Moscow, 2014).
A. G. Chmielewski, T. S. Urbanski, and W. Migdal, Hydrometallurgy 45, 333 (1997). https://doi.org/10.1016/S0304-386X(96)00090-4
M. Ya. Visaliev, M. Ya. Shpirt, Kh. M. Kadiev, V. I. Dvorkin, E. E. Magomadov, and S. N. Khadzhiev, Solid Fuel Chem. 46, 100 (2012)
I. Tsuboi, S. Kasai, E. Kunugita, et al., J. Chem. Eng. Jpn. 24, 15 (1991). https://doi.org/10.1252/jcej.24.15
M. V. Tsygankova, V. I. Bukin, E. I. Lysakova, et al., Tsvetn. Met., No. 1, 21 (2011).
G. A. Lukomskaya, K. Z. Shakirov, L. I. Petrova, et al., RF Patent No. 2334800, Byull. Izobret., No. 27 (2008).
R. Schemel, D. Rodriguez, and R. Salazar, US Patent No. 4539186 (1985).
Yu. L. Mikhailov, Cand. Sci. (Chem.) Dissertation (Omsk. State Univ., Omsk, 2001).
A. M. Tereza, S. P. Medvedev, and V. N. Smirnov, Acta Astronaut. 176, 653 (2020). https://doi.org/10.1016/j.actaastro.2020.03.045
A. M. Tereza, G. L. Agafonov, A. S. Betev, and S. P. Medvedev, Russ. J. Phys. Chem. B 14, 951 (2020). https://doi.org/10.1134/S1990793120060299
Funding
The study was financially supported by the Russian Foundation for Basic Research as part of scientific project no. 18-29-24029-mk and state order no. 0089-2019-0018 (registration number АААА-А19-119-022690098-3). Part of the analyses was carried out on the equipment of the Analytical Center for Collective Use of the Institute of Problems of Chemical Physics, Russian Academy of Sciences and Institute of Petrochemical Synthesis, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salgansky, E.A., Kislov, V.M., Tsvetkov, M.V. et al. Energy Production and Recovery of Rare Metals from Ash Residue During Coal Filtration Combustion. Russ. J. Phys. Chem. B 16, 268–277 (2022). https://doi.org/10.1134/S1990793122020105
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793122020105