Abstract
In an experiment to study radiation “bystander effects” (BEs) at the interorganismic (systemic) level, we use mice irradiated at a dose of 3 Gy and mice that are not irradiated, bystanders, kept together. In the irradiated animals, on the 3rd and 60th days, when kept without a partition, and on the 14th day when kept in cells with and without a partition, a statistically significant decrease in the frequency of normal chromatophilic (oxyphilic) erythrocytes (NCEs) with micronuclei is revealed when compared with the gamma control. In the nonirradiated bystander-mice, kept with the irradiated mice in a cage with a partition on the 14th day and in a cage without a partition on the 60th day, a tendency to exceed the micronucleus (MN) frequency of the erythrocytes’ values in the biocontrol is revealed. Based on the data obtained in this experiment, it is assumed that the radiation BE has the opposite character; i.e., nonirradiated organisms are able to reduce the radiation effects in irradiated individuals: the “rescue effect.”
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
It has now been established that the biological effects in cells after exposure to ionizing radiation (IR) are caused not only by damage to the nuclear DNA. Cells that are near the irradiated cells or in the culture medium obtained from them can also exhibit changes similar to those found in the irradiated cells. The so-called non-targeted effects of IR, including the “bystander effect” (BE), have been fairly well studied in vitro and in vivo [1, 2]. It was shown that BE is characterized not only by the transfer of damage from irradiated cells to nonirradiated ones in the form of chromosomal aberrations, gene mutations, and changes in gene expression but also by apoptosis, the neoplastic transformation of the cells of the bystanders. It should be noted that together with the cytotoxic effect, cytoprotective effects, for example, an increase in their radio resistance, are also recorded in the cells of bystanders.
Previously, it was believed that BE could only be realized within one organ or system. Recently it was shown that BE could be recorded not only in cell cultures or in vivo in one organism but also at the interorganismic level: an irradiated organism can induce changes in an unirradiated organism. Such effects have been found in invertebrates (Daphnia magna) [3] and higher vertebrates—fish [4, 5] and rats [6]—and were called radiation-induced interorganismic level bystander effects [3]. Several mechanisms are proposed to explain this phenomenon: (1) direct contact and transmission of damaging factors; (2) the isolation of compounds that can cause changes in nonirradiated animals at a distance [7]; (3) the action of biophotons [8]; and (4) the action of extracellular DNA [9].
The distant BE has been least studied in terms of the manifestations and mechanisms of the phenomenon related to nontargeted effects. In the works [10, 11], the first results of research and observation in a comprehensive project to assess radiation-induced nontargeted effects are presented. In our previous experiment, nonirradiated mice that were in contact with irradiated (3 Gy) individuals for 3 months showed signs of pathologies similar to radiation-induced ones: a change in behavioral reactions, a statistically significant tendency toward a decrease in the weight of the spleen (r = –0.416, p = 0.048), an increase in the area of alopecia (r = –0.631, p = 0.001), and other anomalies.
Under the influence of IR, irradiated mammalian cells are able to transmit extracellular signals to the nonirradiated neighboring cells. The work [12] also describes a phenomenon called the rescue effect, in which the bystander cells reduced the radiation-induced changes in irradiated cells using the intercellular feedback signal.
This topic can be applied in the environmental assessment of the impact of radioactivity on humans and natural resources [13–15]. The aim of our experiment is to study radiation bystander effects at the interorganismic level in irradiated and nonirradiated animals (mice) when they are kept together.
MATERIALS AND METHODS
The study was carried out on 60 outbred female mice. The experiment used healthy animals that had not previously been subjected to other experimental effects. The groups were formed by the method of continuous sampling. During the experimental procedures, the relevant international rules for working with laboratory animals were observed [16, 17]. Animals (n = 20) were irradiated at a dose of 3 Gy at the IGUR-1M research radiobiological gamma facility with four isotope sources 137Cs, the gamma dose rate was 0.79 Gy/min, and the unevenness of the γ-field in the workspace did not exceed 5%. The absorbed dose for mice was 3.0 Gy.
After the treatment, the mice were placed in groups of four cages, each of which contained five irradiated mice and five nonirradiated mice (bystander mice, n = 20). In two of the four cages, irradiated and non-irradiated mice could freely contact each other. In the other two cells, mice in the irradiated and nonirradiated groups were separated by a metal net, which prevented their tactile contact. Two more groups were used for the control: the biocontrol group (n = 10) and the group of the gamma control animals irradiated at a dose of 3 Gy, (n = 10), each of which was contained in a separate cell. In total, six experimental groups were formed. The experiment lasted 3 months.
The analysis of the frequency of the micronuclei (MN) of the erythrocytes was applied to solve the set tasks. The MN test is an effective tool for assessing the genotoxic effect of various factors, disorders related to genome instability, and other cellular pathological processes related to damage to the DNA and chromatin, and related to impaired cell division [18]. Blood was collected from the tail vein of mice on the 3rd, 7th, 14th, 30th, 60th, and 90th days after the start of the experiment. Blood smears were prepared to determine the frequency of erythrocytes with micronuclei and the cellular composition of peripheral blood. The smears were fixed with methanol for 3 min, dried, and stained by the Romanovsky–Giemsa method. The slides for analysis were coded and then analyzed using Axio Scope A1 and Axio Imager M2 microscopes (Carl Ziess, Germany). The maximum single blood loss for each animal did not exceed 3% of the circulating blood volume (for mice of this age group). To determine the frequency of erythrocytes with micronuclei, 1000 polychromatophilic erythrocytes (PCEs) and at least 2000 normal chromatophilic (oxyphilic) erythrocytes (NCEs) were analyzed.
Statistical analysis was performed using Student’s t-criterion. Differences were considered statistically significant at the probability of the null hypothesis p < 0.05. For a comparative analysis of the data obtained, the following influencing factors were identified: gamma irradiation; time elapsed after the start of the experiment; contact factor, i.e., the type of contact between irradiated and nonirradiated animals (0 indicates no contact between irradiated and nonirradiated animals; 1 indicates olfactory contact (cage with a partition); and 2 indicates tactile and olfactory contact (cage without a partition)). Multifactor variance analysis for the signs of contingency was carried out using a generalized linear model.
RESULTS AND DISCUSSION
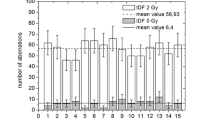
The studies evaluated the frequency of PCEs with micronuclei, NCEs with micronuclei, and the total number of erythrocytes in the peripheral blood in mice on the 3rd, 7th, 14th, 30th, 60th, and 90th days after the start of the experiment. The average frequency of PCEs with micronuclei in the peripheral blood of mice from the biological control group was 0.2–0.6‰. The gamma irradiation of animals resulted in a statistically significant (t = 2.95, p = 0.01) increase in the frequency of PCEs with micronuclei, starting from the seventh day after irradiation, when this indicator exceeded the value in the biological control group by almost 5 times. This high level of cytogenetic damage persisted for up to 14 days, followed by a decrease in the frequency of chromosomal damage (Fig. 1).
In groups of nonirradiated bystander mice, no statistically significant changes in the frequency of PCEs with micronuclei were found. On the 7th day, in the group of bystander mice kept with irradiated mice in a cage with a partition, a tendency toward an increase in the frequency of the MN of the erythrocytes was revealed (t1 = 0.88, p = 0.39; Fig. 2). A significant (t = 2.32, p = 0.03) decrease in the frequency of PCEs with micronuclei compared to the value in the gamma control group (Fig. 3) was recorded in the group of irradiated animals kept in the same cage with a partition with nonirradiated witness mice on day 14 after the start of the experiment. 3). When comparing the frequency of PCEs with micronuclei in groups of irradiated animals that were kept together with nonirradiated bystanders, no other significant changes were found.
Frequency of MN of PCEs depending on the time after irradiation for groups of animals: (1) biocontrol; (2) bystanders having had tactile contact with irradiated animals; (3) bystanders in contact with irradiated mice through the partition; (4) total values (control). On the 7th day, in the group of bystanders kept with irradiated mice in a cage with a partition, a tendency toward an increase in the frequency of the MN of erythrocytes (t1 = 0.88, p = 0.39) was revealed.
Frequency of MN of PCEs depending on the time after irradiation for groups of animals: (1) gamma control; (2) irradiated animals, having had tactile contact with bystanders; (3) irradiated mice in contact with bystanders through the partition; (4) the total number of micronucleated erythrocytes in the gamma control (polychromatophilic + normochromatophilic). On the 14th day, a statistically significant (t = 2.32, p = 0.03) decrease in the frequency of MN erythrocytes in irradiated mice in contact with the bystanders through the partition.
When conducting multivariate variance analysis in the main generalized model, a significant effect on the frequency of PCEs with micronuclei of the radiation factor (F = 59.45, p = 1.3 × 10–13) and time after the start of the experiment (F = 4.58, p = 0.033; Tables 1, 2) was revealed. At the same time, gamma irradiation led to an increase in the frequency of PCEs with micronuclei by an average of 0.97‰, and the indicator decreased by 0.0042‰ per day.
When analyzing the frequency of NCEs with micronuclei in the biological control group, it was found that this indicator at the beginning of the experiment was (1.09 ± 0.18)‰. Subsequently, it decreased, and on the 90th day after the start of the experiment in the biological control group, the frequency of NCEs with micronuclei was 0.26 ± 0.12‰. The gamma irradiation of animals at a dose of 3 Gy led to a statistically significant increase in the frequency of chromosomal aberrations at all periods of the examination. On the 14th day after the start of the experiment, the value of this indicator was 3.7 times higher than its level in the biological control group. Further, from the 30th day to the 90th day after the beginning of the experiment in the gamma control group, the frequency of NCEs of erythrocytes with micronuclei did not change significantly and amounted to about 1‰, which exceeded the values in the biological control group by 2–3 times (Fig. 4).
In the groups of nonirradiated bystander animals, no statistically significant changes in the frequency of NCEs with micronuclei were found in comparison with the values of the indicator in the biological control group (Fig. 5). In the groups where the irradiated mice were kept with nonirradiated bystanders, the irradiated animals showed a statistically significant decrease in the frequency of NCEs with micronuclei when compared with the gamma control t2 (Fig. 6). An indicator of comparison with biocontrol t1, where p is the level of significance, is also given.
Dependence of the frequency of MN normochromatophilic erythrocytes (NCE) on the time elapsed after irradiation for groups of animals: (1) biocontrol, and (2) bystanders having had tactile contact with irradiated animals; (3) bystanders having had contact with the irradiated mice through the partition.
In the irradiated mice, there was a significant decrease in the frequency of NCEs with micronuclei as follows:
(1) on the 3rd day after the start of the experiment when kept in one cage without a partition, t1 = 0.39 and p = 0.701; and t2 = 2.35 and p = 0.03;
(2) on the 14th day, as in the group of animals kept in cages without a partition, t1 = 1.72 and p = 0.1; and t2 = 6.03 and p = 0.000011; and in the group in cells with a partition, t1 = 4.28 and p = 0.0005; and t2 = 3.29 and p = 0.004.
At the same time point (on the 14th day), in nonirradiated bystander mice kept with irradiated mice in a cage with a partition, a tendency toward an excess of the frequency of erythrocytes of indices in biocontrol was revealed (t1 = 1.79, p = 0.09; Fig. 5);
(3) on the 60th day in the group where the animals were kept in cages without a partition, t1 = 0.75, p = 0.46; and t2 = 2.8, p = 0.01.
Also on the 60th day, in non-irradiated bystanders mice, which were with irradiated mice in a cage without a partition, a tendency was revealed for an excess of the frequency of the MN of the erythrocytes of the indicators in the biocontrol (t1 = 1.39, p = 0.18; Fig. 5).
An increase in the frequency of NCEs with micronuclei in the peripheral blood was also revealed in irradiated animals that were kept with nonirradiated bystanders in the same cage with a partition (group 2) on the 30th day after the start of the experiment (Fig. 6).
The multivariate analysis revealed a significant effect of the following factors: irradiation, time elapsed after start of experiment, and contact factor (Tables 3, 4).
The frequency of all erythrocytes with micronuclei is actually a combination of the data for the frequency of PCEs and NCEs with micronuclei; however, due to the increase in the number of red blood cells analyzed, in this case, the statistical power of the analysis increases (Tables 5, 6). When irradiated and nonirradiated mice are kept in one cage without a partition, a decrease in the frequency of erythrocytes with micronuclei by an average of 0.43‰ is recorded in the irradiated mice.
CONCLUSIONS
The effect of IR led to an increase in the frequency of erythrocytes with micronuclei in the peripheral blood of mice, and the indicator decreased depending on the time that had elapsed after the start of the experiment. Tactile and olfactory contact led to a decrease in the frequency of NCEs with micronuclei in irradiated mice, which were kept in cages with nonirradiated bystanders. The irradiated animals showed a statistically significant decrease in the frequency of NCEs with micronuclei when compared with the gamma control t2 as follows: (1) on the 3rd day after the start of the experiment when the mice were kept in one cage without a partition, t2 = 2.35 and p = 0.03; (2) on the 14th day as in the group where the animals were kept in a cage without a partition, t2 = 6.03 and p = 0.000011; and in the group of animals in cages with a partition, t2 = 3.29 and p = 0.004; (3) on the 60th day in the group where the animals were kept in cages without a partition, t2 = 2.8 and p = 0.01.
At the same time, there was a tendency toward an increase in the frequency of the MN of the NCEs in unirradiated bystander mice. On the 14th day, these bystander mice, kept with irradiated mice in a cage with a partition, showed a tendency toward an excess of the frequency of the MN of the erythrocytes in the biocontrol parameters (t1 = 1.79, p = 0.09).
Analysis of the published data in the study of the bystander effect at the cell level [9] showed the role of extracellular DNA in the formation of radiation effects in nonirradiated cells. According to A.V. Ermakova et al., the chain of sequential events in the signaling system is as follows: irradiation → primary oxidative stress → DNA modification → apoptosis of damaged cells → free modified extracellular DNA → signal reception by nonirradiated cells → secondary oxidative stress → DNA modification, etc. A radiation-induced BE involving the DNA signaling pathway was observed in undifferentiated and differentiated human cells forming suspension or monolayer cultures.
The discussion of the possible mechanisms of the radiation bystander effect at the level of the organism from the point of view of biochemistry was linked by the authors with the theory of radiotoxins by A.M. Kuzin [19, 20]. We have made an assumption about the important role of lipoperoxide derivatives in the formation of the studied effect. Lipoperoxides formed as a result of exposure to ionizing radiation on the body are highly toxic volatile compounds [21]. The products of lipid peroxidation and the appearance of volatile metabolites can cause the appearance of a molecular signal leading to a change in the cells of unirradiated bystanders’ organisms [10, 11].
In a series of experiments conducted by a group of Canadian researchers at McMaster University [4–6], as an explanation of the mechanisms of the bystander effect, the hypothesis about the role of ultraviolet biophotons in the transmission of the bystander signal between organisms is being tested, and some of the provisions of this theory have already been confirmed. It is assumed that the use of these principles of quantum biophysics will bring the discussion of the mechanism of the phenomenon to a fundamentally new level [5, 6].
The phenomenon of the rescue effect is also known from these foreign sources. The effect was observed in both primary human fibroblast (NHLF) and cancer cells (HeLa) using two-cell coculture systems. After the cocultivation of irradiated cells with nonirradiated bystander cells for 24 h, the number of foci of the p53-binding protein 1, corresponding to the number of DNA double strand breaks in irradiated cells, was less than in the irradiated cells that were not cocultured with the cells of the bystanders (0.78 ± 0.04 versus 0.90 ± 0.04 foci/cell) at a statistically significant level. Similarly, the formation of micronuclei and the degree of apoptosis in irradiated cells differed at statistically significant levels if they were cocultured with bystander cells [12].
Based on the data obtained in the present experiment and the results of studies of other authors of the bystander effect under exposure to radiation, it can be assumed that nonirradiated organisms are able to reduce the radiation effects in exposed individuals. At the organismic (systemic) level, the combination of the effects of DNA modification with subsequent apoptosis, as well as other cellular phenomena, and the formation of volatile metabolites that affect blood cells induce BE, the reverse side of which is the rescue effect. The results indicate the need for further studies of the radiation-induced bystander effect using molecular-cell methodological approaches.
REFERENCES
C. Mothersill, A. Rusin, C. Fernandez-Palomo, and C. Seymour, Int. J. Radiat. Biol. 94, 696 (2018). https://doi.org/10.1080/09553002.2017.1398436
I. I. Pelevina, V. V. Petushkova, V. A. Biryukov, et al., Rad. Biol. Radioekol. 59, 261 (2019). https://doi.org/10.1134/S086980311903010X
P. Reis, J. Lourenco, F. P. Carvalho, et al., Aquat. Toxicol. 198, 206 (2018). https://doi.org/10.1016/j.aquatox.2018.03.007
C. Mothersill, R. W. Smith, R. Saroya, et al., Int. J. Rad. Biol. 86, 817 (2010). https://doi.org/10.3109/09553002.2010.486018
C. Mothersill, R. Smith, J. Wang, et al., Dose-Response 16, 1559325817750067 (2018). https://doi.org/10.1177/1559325817750067
R. Smith, J. Wang, C. Seymour, et al., Dose Response 16, 1559325817750068 (2018). https://doi.org/10.1177/1559325817750068
B. P. Surinov, N. N. Dukhova, and V. G. Isaeva, Radiats. Risk 24 (3), 105 (2015).
C. Mothersill, A. Rusin, and C. Seymour, Environ. Res. 159, 484 (2017). https://doi.org/10.1016/j.envres.2017.08.029
A. V. Ermakov, M. S. Kon’kova, S. V. Kostyuk, and N. N. Veiko, Rad. Biol. Radioekol. 51 (6), 1 (2011).
V. V. Petushkova, I. I. Pelevina, I. N. Kogarko, et al., Rad. Biol. Radioekol. 60, 229 (2020). https://doi.org/10.31857/S0869803120030108
V. V. Petushkova, I. I. Pelevina, I. N. Kogarko, et al., Biol. Bull. 47, 1610 (2020). https://doi.org/10.1134/S1062359020120079
S. Chen, Y. Zhao, W. Han, et al., Mutat. Res. 706, 59 (2011). https://doi.org/10.1016/j.mrfmmm.2010.10.011
S. O. Travin, Yu. I. Skurlatov, and A. V. Roshchin, Russ. J. Phys. Chem. B 14, 86 (2020).
E. V. Shtamm, Yu. I. Skurlatov, A. V. Roshchin, V. O. Shvydkii, L. V. Semenyak and I. V. Semenova, Russ. J. Phys. Chem. B 14, 122 (2020).
Yu. I. Skurlatov, E. V. Shtamm, L. N. Shishkina, A. V. Roshchin, V. O. Shvydkii and L. V. Semenyak, Russ. J. Phys. Chem. B 14, 130 (2020).
Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes.
European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes ETS No. 123, Appendix A.
M. Hayashi, Genes. Environ. 38, 18 (2016). https://doi.org/10.1186/s41021-016-0044-x
Radiotoxins, their Nature and Role in the Biological Action of High Energy Radiation, Collection of Articles, Ed. by A. M. Kuzin (Atomizdat, Moscow, 1966) [in Russian].
A. M. Kuzin and V. A. Kopylov, Radiotoxins (Nauka, Moscow, 1983) [in Russian].
E. A. Neifakh, Vopr. Med. Khim. 23, 131 (1977).
ACKNOWLEDGMENTS
The authors thank Candidate of Biological Sciences E.I. Selivanova and Candidate of Medical Sciences N.S. Kuzmina for their participation in the preparation of this article.
Funding
This study was carried out as part of a state assignment of the Ministry of Education and Science of Russia through a subsidy allocated by the Federal Research Center for Chemical Physics, Russian Academy of Sciences, topic 0082-2019-0015 “Study of the principles of the structural and functional organization of biomolecular systems, the development of design methods for their physicochemical analogs, and the creation based on this of biologically active drugs of a new generations,” state registration no. AAAA-A20-120031490003-7; and this study was also partially supported by the Russian Foundation for Basic Research, grant 16-04-00963/18.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pelevina, I.I., Akleev, A.V., Kogarko, I.N. et al. Radiation-Chemical Effect of Ionizing Radiation on the Organism and Genotoxic Disorders of the Blood System. Russ. J. Phys. Chem. B 15, 1046–1053 (2021). https://doi.org/10.1134/S1990793121060233
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793121060233