Abstract
Based on thermodynamic calculations, an assessment is made of the modes of the noncatalytic conversion of natural gas to produce synthesis gas. The following modes of conversion are considered: air and steam-air, with an increased oxygen content and an increased initial temperature of the mixture. The results of calculations for mixtures with an adiabatic combustion temperature not lower than 1000 K are presented, since at lower temperatures unreacted methane appears in the products. It is shown that when air is used as an oxidizing agent, the maximum content of hydrogen and carbon monoxide in gaseous products can be 28.2 and 13.2 vol %, respectively, at the equivalence ratio φ = 2.6. In this case, the maximum yields of hydrogen and carbon monoxide are 1.65 and 0.77 moles from 1 mole of methane, respectively. In the case of the steam-air conversion of methane, the maximum yield of hydrogen and carbon monoxide is less than in the case of air conversion. In the conversion of methane with an increased oxygen content, the maximum hydrogen yield is lower and that of carbon monoxide is higher than in the conversion of air. An increase in the initial temperature of the mixture leads to an increase in the adiabatic combustion temperature and the yield of carbon monoxide, as well as to a decrease in the yield of hydrogen in the products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Natural gas, which is 80–90% methane, is an extremely important raw material for the chemical industry, as it is one of the main sources of synthesis gas production. The latter is used for the production of methanol, ammonia, and pure hydrogen [1–3]. However, the production of synthesis gas requires high energy costs and is carried out mainly by reforming light hydrocarbons or by the steam-oxygen gasification of coal [4, 5]. A fairly large number of methods for producing synthesis gas by partial oxidation of hydrocarbons, primarily natural gas, are known [6–8]. This technology has become widespread due to its low cost: it is cheaper by factors of 2 to 3 than obtaining hydrogen by the electrolytic decomposition of water [9]. In addition to natural gas, synthesis gas can also be obtained by gasification of both liquid [10] and solid fuels [11, 12].
Due to the fact that recently hydrogen has been considered as a promising environmentally friendly energy carrier, there is a need to create energy-efficient large-scale production of hydrogen to meet the needs and infrastructure of hydrogen energy [13]. The cheapest way to produce hydrogen is from natural gas (blue hydrogen); however, the existing technologies do not always provide an acceptable depth of hydrogen recovery and are complicated due to the use of rapidly coking catalysts. The most attractive developed method for producing hydrogen from hydrocarbon gases is the process of noncatalytic partial oxidation [14], which has a number of advantages over catalytic methods and has only thermodynamic limitations.
In [15], a thermodynamic analysis of synthesis gas production by the partial oxidation of biogas was carried out. The influence of various parameters has been investigated, such as the temperature, pressure, fuel ratio, and CO2 content, for the production of synthesis gas and the formation of soot. Increasing temperature has been shown to play a key role in improving product quality and reducing soot formation. This study proposes the optimal operating mode to reduce the adverse effects of pressure increases and achieve the desired amounts of hydrogen and carbon monoxide. In [16], the authors analyzed the results of the thermodynamic calculation of the steam reformation of methane by minimizing the Gibbs free energy to produce hydrogen or synthesis gas in the ranges of the steam/methane ratio from 0.5 to 3, operating pressure from 1 to 50 bar, and temperature in the reaction zone from 600 to 1200 K. It was shown that in order to achieve the maximum ratio of Н2 relative to CO, the temperature in the reaction zone should exceed 900 K, and the ratio of H2O/CH4 should be in the high range.
A comparison of the results of the numerical simulation of reforming steam under conditions of thermodynamic equilibrium with the experimental data is presented in [17]. The analysis showed a decrease in the energy efficiency of reforming hydrocarbon with an increase in the carbon number. It has been shown that the efficiency of reforming diesel fuel is at least 15% lower than that of natural gas. In [18], a detailed thermodynamic analysis of the chemical reformation of methane with increased sorption was carried out using CaO and NiO as the CO2 sorbent and oxygen transfer material, respectively. The presence of CaO leads to a higher conversion of methane at low temperatures. The addition of NiO minimizes the thermal requirements and results in better performance than using pure oxygen as an oxidizer or purge gas. The steam reforming process of biogas, in which this reaction and the adsorption of CO2 occur in one device, was proposed in [19]. The results showed that the concentration of H2 in the products of reforming steam, biogas increases with an increase in operating temperatures and the steam/methane ratio. In [20], thermodynamic analysis was used to study and compare the characteristics of hydrogen production reactions in one-stage (autothermal methane reformation (ATR)) and two-stage (partial methane oxidation followed by water gas reactions (POM + WGSR) processes. A comparison of the ATR and POM processes in combination with WGSR based on the condition of the stoichiometric ratio CH4 : O2 : H2O = 1: 0.5 : 1 shows that the two-stage process provides higher productivity in terms of Н2.
According to the results of studies in the field of hydrogen production from natural gas in the filtration combustion modes, it can be concluded that when air is used as an oxidizer, the maximum hydrogen content in gaseous products can be 34 vol % [21]. Moreover, in most works, the maximum hydrogen content in the products was ~25 vol % [14, 22, 23]. It is possible to increase the concentration of hydrogen in products by using catalysts and/or an oxygen-enriched oxidizer. The use of catalysts makes it possible to significantly increase the hydrogen concentration in products, which can reach 80 vol % in the case of catalytic methane reformation [24]. However, the latter is accompanied by the “poisoning” of the catalyst surface with carbon deposits and, consequently, their subsequent regeneration. The use of an oxygen-enriched oxidizer in the reformation, in turn, is accompanied by significant energy consumption for its production [25, 26], and imposes stringent requirements on the safety and construction materials for creating equipment.
To optimize the methods for producing a hydrogen-containing gas by the method of noncatalytic conversion (partial oxidation) of natural gas, it is necessary to simulate this process. The aim of this study is a thermodynamic assessment of the optimal conditions for the production of hydrogen and synthesis gas during the noncatalytic conversion of natural gas at atmospheric pressure. The paper compares two conversion modes: with the joint and separate feeding of reagents.
CALCULATION PROCEDURE
Calculations using thermodynamic equilibrium models to study the behavior of complex chemical systems are often used and described in the industrial and scientific literature [27, 28]. The establishment of the phase and chemical equilibrium in each system is an irreversible process and, in accordance with the second law of thermodynamics, is characterized by an increase in entropy. Thus, the calculation of the equilibrium parameters of isolated multicomponent thermodynamic systems is simplified and reduced to the problem of establishing a state characterized by the maximum entropy. The approach to calculate the thermodynamic equilibrium by searching for the minimum of the Gibbs free energy of the system is also used [16–20]. These approaches are equivalent. To determine the conditions for obtaining a combustible gas with the maximum content of hydrogen and carbon monoxide, a thermodynamic analysis of the conversion of natural gas was carried out using the program for calculating high-temperature thermochemical equilibria TERRA [27] while varying the composition and enthalpy of the mixture.
We considered a system consisting of methane, nitrogen, and oxygen, with different contents of water vapor ([H2O]/[O2] = 0, 0.5, 1). The consumption of methane in the mixture was fixed (1 g/s), and the equivalence ratio φ, which is equal to the ratio of the amount of oxygen required for complete oxidation of methane to the amount of oxygen supplied, varied from 1 to 2.6. Rich fuel mixtures have a stoichiometric ratio greater than one, values φ < 1 correspond to lean mixtures, and φ = 1 corresponds to the complete oxidation of methane into carbon dioxide and water. The calculations were carried out at atmospheric pressure (0.1 MPa). We changed the initial temperature of the reagents and, accordingly, the enthalpy, which was calculated according to Kirchhoff’s law. For all values of the initial temperature, it was conventionally assumed that water is in a gaseous state. As a result of the calculations, the equilibrium composition of the products and the value of the adiabatic combustion temperature were obtained. The paper does not present the results of calculations with an adiabatic combustion temperature below 1000 K. The rate of the chemical reaction has a strong dependence on temperature, and at low temperatures, thermodynamic equilibrium is established for a rather long time [29, 30].
To assess the conversion efficiency, the molar yield of hydrogen and CO from 1 mol of methane was calculated. From 1 mol of methane, the maximum yield of hydrogen can be 2 mol; and of carbon monoxide, 1 mol.
RESULTS AND DISCUSSION
Conversion of Natural Gas with Air and Steam
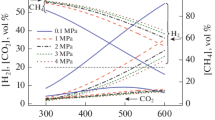
The initial thermodynamic calculations were carried out for the simplest case of conversion of methane with air: without adding water vapor. Figure 1 shows the calculation data for the composition of the products and the adiabatic combustion temperature during the conversion of methane with air without adding water vapor.
The hydrogen content increases linearly with increasing φ: from 0 at φ = 1 to 28.2 vol % at φ = 2.6 (Fig. 1). The content of carbon monoxide depends logarithmically on the value of φ and, with its increase, changes from 0 to 13.2 vol %. With an increase in φ, the volume fraction of the water vapor in the products decreases from 18.8 to 5.7%, and the concentrations of nitrogen and carbon dioxide also decrease. With an increase in φ, the adiabatic combustion temperature decreases almost linearly from 2148 to 1005 K. The volume ratio of hydrogen to carbon monoxide varies from 0.4 to 2.1 with increasing φ.
The maximum yields of hydrogen and carbon monoxide are 1.65 and 0.77 mol, respectively, at φ = 2.6. With this value φ, the products contain unreacted methane in an amount of 0.17 vol %, which confirms the ineffectiveness of conversion at temperatures below 1000 K.
When steam is added to the initial mixture in the ratio [H2O]/[O2] = 0.5 mol/mol in comparison with the case without steam, the adiabatic temperature decreases by 250–400 K at similar values of φ (Fig. 2). In the steam-air conversion of methane, the hydrogen content increases linearly with an increase in φ from 0 to 23.2 vol %. With an increase in the value of φ, the volume fraction of water vapor decreases from 26.1 to 12.6%, and the concentrations of nitrogen and carbon dioxide also decrease. With an increase in φ, the CO content in the products increases from 0 to 8.5 vol %. The volumetric hydrogen/carbon monoxide ratio varies from 0.8 to 2.7 with increasing φ. In this case, the maximum amount of hydrogen is observed at lower values of φ (taking into account T > 1000 K), namely, for φ = 2.2 [H2] = 23.2 vol % compared to air conversion.
The maximum yields of hydrogen and carbon monoxide are 1.58 and 0.58 mol, respectively, at φ = 2.2. At this φ value, the products contain unreacted methane of 0.02 vol %.
The calculation of the material balance showed that the content of water vapor in the products exceeds their initial content by about 2–3 times. In the case of the steam-air conversion of methane in the case of [H2O]/[O2] = 1 mol/mol, the hydrogen content increases linearly with an increase in φ from 0 to 16.5 vol % (Fig. 3). With an increase in the value of φ, the volume fraction of water vapor in the products decreases from 32.0 to 21.1%. The dependence of the carbon monoxide concentration on φ is similar to the previous cases, with the CO content increasing from 0 to 4.7 vol % with increasing φ. For [H2O]/[O2] = 1 the decrease in adiabatic temperature is approximately 300 K for similar values of φ at [H2O]/[O2] = 0.5. At φ = 1.8, the products contain unreacted methane in the amount of 0.002 vol %.
The maximum yields of hydrogen and carbon monoxide are 1.36 and 0.39 mol, respectively, at φ = 1.8. Thus, it can be concluded that at a combustion temperature of at least 1000 K, the maximum yield of hydrogen and carbon monoxide is realized for the case of the air conversion of methane.
The content of water vapor in the products exceeds their initial content by approximately 1.5–2 times. With an increase in the content of water vapor in the initial reagents, their volume concentration in the products increases, and the nitrogen concentration decreases (see Figs. 1–3).
However, it is not necessary to directly compare the values of the volume concentration of hydrogen in the products for different mixtures, since the initial composition of the reagents changes. In the case of adding water vapor, for example, for a mixture with a fixed value φ = 1.67, with an increase in the molar ratio [H2O]/[O2] from 0 to 1.5, the hydrogen yield increases from 0.87 to 1.39. A further increase in the water vapor content leads to the temperature dropping significantly below 1000 K, a decrease in the hydrogen yield, and the appearance of unreacted methane in the products. With an increase in the water vapor content, the hydrogen yield passes through the maximum value. The adiabatic temperature and the carbon monoxide content decrease monotonically with an increase in the water vapor content.
The maximum hydrogen content in the mixture at a temperature of at least 1000 K is achieved in the following cases:
(1) for φ = 2.6 and [H2O]/[O2] = 0, it is 28.2 vol %;
(2) for φ = 2.2 and [H2O]/[O2] = 0.5, it is 23.2 vol %;
(3) for φ = 1.8 and [H2O]/[O2] = 1, it is 16.5 vol %.
We will take these compositions as basic and carry out additional optimization of the synthesis gas yield.
Conversion of Natural Gas with Oxygen-Enriched Air and Steam
The main effect of air enrichment with oxygen during the conversion of natural gas is an increase in the temperature of the process and an increase in the concentration of the synthesis gas components: H2 and CO. However, in practice, there is a temperature limitation from above, related to the resistance of refractory materials; and from below, related to the incomplete consumption of methane and the formation of soot under equilibrium conditions [31].
We consider the case [H2O]/[O2] = 0. For these cases, the values of φ were fixed, i.e., with an increase in the oxygen concentration, the methane concentration increased and the nitrogen concentration in the initial mixture decreased.
With an increase in the oxygen concentration in the mixture from 21 to 41 vol %, there is a linear increase in the hydrogen concentration from 28.2 to 38.8 vol %; and in the carbon monoxide concentration from 13.2 to 21.2 vol % (Fig. 4). The adiabatic combustion temperature with increasing [O2] grows almost linearly from 1005 to 1285 K. The carbon dioxide content does not depend on the initial oxygen concentration and is approximately 3–4 vol %. The content of water vapor in the products increases, and the nitrogen content decreases with an increase in the initial oxygen concentration.
Dependences of the adiabatic combustion temperature (T) and volumetric content of gases (V) on the content O2 for φ = 2.6 and [H2O]/[O2] = 0. The designations are the same as in Fig. 1.
An increase in the combustion temperature with an increase in the oxygen content in the gas allows the use of richer mixtures, which, when converted with air, have adiabatic temperatures below 1000 K. For example, with an oxygen content of 31 vol %, the conversion of methane with φ = 2.9 makes it possible to obtain a gas with a hydrogen content of 38.0 vol % and a carbon monoxide content of 18.1 vol %; the combustion temperature is 1000 K. The maximum yields of hydrogen and carbon monoxide are 1.62 and 0.84 mol, respectively.
At an oxygen content of 41 vol %, the conversion of methane with φ = 3.0 makes it possible to obtain a gas with a hydrogen content of 44.3 vol % and a carbon monoxide content of 21.4 vol %; the combustion temperature is 1021 K. The maximum yields of hydrogen and carbon monoxide are 1.58 and 0.87 mol, respectively.
Let us consider the case of the steam-air conversion of methane at φ = 1.8 and [H2O]/[O2] = 1 with an increased amount of oxygen in the mixture (Fig. 5). With an increase in the oxygen concentration from 21 to 41 vol %, the hydrogen concentration increases similarly to the previous case (see Fig. 4). The maximum amount of hydrogen is 20.7 vol %. The concentration of carbon monoxide increases from 4.7 to 13.6 vol %. The adiabatic combustion temperature with increasing [O2] for φ = 1.8 and [H2O]/[O2] = 1 grows linearly from 1039 to 1662 K. As in the previous case (see Fig. 4), the carbon dioxide content does not depend on the initial oxygen concentration. The content of the water vapor in the products increases, and the nitrogen content decreases with an increase in the initial oxygen concentration.
An increase in the combustion temperature with an increase in the oxygen content in the gas allows the use of richer mixtures. At an oxygen content of 31 vol %, the methane conversion with φ = 2.2 and [H2O]/[O2] = 1 allows us to obtain gas with the hydrogen and carbon monoxide content of 28.3 and 8.3 vol %, respectively; the combustion temperature is 1006 K. The maximum yields of hydrogen and carbon monoxide are 1.26 and 0.5 mol, respectively.
At an oxygen content of 41 vol %, the methane conversion with φ = 2.4 and [H2O]/[O2] = 1 allows us to obtain gas with the hydrogen and carbon monoxide content of 35.3 and 10.8 vol %, respectively; the combustion temperature is 1007 K. The maximum yields of hydrogen and carbon monoxide are 1.2 and 0.57 mol, respectively. Thus, it can be concluded that at a combustion temperature of at least 1000 K, the maximum hydrogen yield decreases, and the carbon monoxide yield increases in the case of air enrichment with oxygen.
An increase in oxygen in the initial mixture at a fixed value of the ratio [H2O]/[O2] leads to an increase in the vapor content. However, as shown in the previous section, the addition of steam leads to a decrease in the concentrations of hydrogen and carbon monoxide. To optimize the resulting gas mixture with respect to hydrogen during steam conversion of methane, additional thermodynamic calculations were performed at an increased oxygen content. We considered mixtures with the values of the ratio [H2O]/[O2] = 0.5 and φ = 2.2, as well as [H2O]/[O2] = 1 and φ = 1.8. In these cases, with an increase in the oxygen content, we did not fix the value of [H2O]/[O2], and the initial amount of steam was fixed; therefore, the ratio [H2O]/[O2] decreased in proportion to the increase in [O2]. The content of the main combustible gases and the adiabatic combustion temperature depending on the oxygen content in the mixture are presented in Table 1.
For the hydrogen-optimized calculations at φ = 2.2, with an increase in the oxygen concentration in the mixture from 21 to 41 vol %, the hydrogen concentration linearly increases from 23.2 to 30.3 vol %; and the carbon monoxide concentration, from 8.5 to 17.6 vol %. The adiabatic combustion temperature with [O2] increasing from 21 to 41 vol % increases from 1018 to 1474 K.
For the hydrogen-optimized calculations at φ = 1.8, with an increase in the oxygen concentration from 21 to 41 vol %, the increase in the hydrogen concentration is insignificant in comparison with the previous case (φ = 2.2). The maximum amount of hydrogen is 20.8 vol %. The concentration of carbon monoxide increases from 4.7 to 13.6 vol %. The adiabatic combustion temperature with [O2] increasing from 21 to 41 vol % grows from 1039 to 1663 K.
Conversion of Natural Gas at an Increased Initial Temperature of the Reactants
To increase the efficiency of the methane conversion, a method with a separate supply of reagents has been proposed [32]. The separate supply of reagents means that one or two of the reagents (oxidizer, steam, methane) are heated before they enter the combustion zone. We will assume that the entire mixture is heated; i.e., we set the initial temperature above 298 K. The initial reagents are heated during the organization of the energy recovery process [33]. For the optimal compositions, we calculate additional options with an increased initial mixture temperature of 400, 500, and 600 K.
The content and yield of the main combustible gases in the products and the adiabatic combustion temperature depending on the initial temperature of the mixture for the cases of air and steam-air conversion of methane are presented in Table 2. An increase in the initial temperature of the mixture leads to an increase in the adiabatic combustion temperature, concentration, and yield of carbon monoxide in the products. At the same time, the content and yield of hydrogen are reduced. An increase in the initial temperature of the mixture for every 100 K leads to an increase in the adiabatic temperature by approximately the same amount. Moreover, the relationship between the concentrations of the products and the initial temperature of the mixture is rather weak.
Figure 6 shows the dependence of the limiting value of the equivalence ratio (φ*) on the initial temperature of the mixture for the cases of air (line 1) and steam-air (line 2) conversion of methane. The limiting value is understood as the value of φ* at which the adiabatic temperature is 1000 ± 5 K. With an increase in the initial temperature of the mixture, the adiabatic combustion temperature rises (see Table 2), which allows the use of richer mixtures, for which the combustion temperature will not be lower than 1000 K. The graph shows that with an increase in the initial temperature of the mixture, the value of φ* at which the combustion temperature is 1000 K also increases. For the initial temperature of 1000 K, a zero reaction effect is observed; i.e., the released heat of exothermic reactions was completely spent on absorption in the endothermic reactions. In the case of the air conversion of methane, the zero reaction effect is realized for a mixture with φ* = 5.2, and in the case of the vapor-air conversion ([H2O]/[O2] = 1), with φ* = 3.4.
For mixtures with a limiting equivalence ratio, unreacted methane is observed in the products. Its content ranges from tenths to several percent by volume. Thus, it can be concluded that the conversion of methane with a combustion temperature below 1000 K is impractical.
CONCLUSIONS
This paper considers the following modes of methane conversion: air and steam-air, with an increased oxygen content and with the increased initial temperature of the mixture. The results of calculations for mixtures with an adiabatic combustion temperature not lower than 1000 K are presented, since at lower temperatures unreacted methane appears in the products.
According to the research results, it can be concluded that in the partial oxidation of natural gas with air as an oxidizing agent, the maximum content of hydrogen and carbon monoxide in gaseous products can be 28.2 and 13.2 vol %, respectively, at a value of φ = 2.6. In this case, from 1 mol of methane, the maximum yields of hydrogen and carbon monoxide are 1.65 and 0.77 mol, respectively.
The addition of steam to the gaseous oxidizer leads to a decrease in the process temperature and the volumetric content of carbon monoxide in the products for a fixed value of the equivalence ratio. With an increase in the vapor content in the initial mixture, the hydrogen concentration increases, reaching the maximum value, after which it begins to decrease. However, in the steam-air conversion of methane for mixtures with the adiabatic combustion temperature not lower than 1000 K, the maximal output of hydrogen and carbon monoxide is less than during air conversion.
By increasing the oxygen concentration during the methane conversion, it is possible to increase the hydrogen and carbon monoxide content, but this increases the combustion temperature. With an increase in the oxygen concentration in the initial mixture from 21 to 41 vol %, there is an almost linear increase in the hydrogen concentration from 28.2 to 38.8 vol % and the carbon monoxide concentration from 13.2 to 21.2 vol % when air is used as the oxidizing agent at φ = 2.6. In this case, the combustion temperature increases by 280 K.
The increase in the combustion temperature with an increase in the oxygen content in the gas allows the use of richer mixtures, which are characterized by low temperatures during conversion with air. For example, at an oxygen content of 31 vol %, the conversion of methane with φ = 2.9 makes it possible to obtain a gas with a hydrogen and carbon monoxide content of 38.0 and 18.1 vol %, respectively; the combustion temperature is 1000 K. The maximum yields of hydrogen and carbon monoxide are 1.62 and 0.84 mol, respectively.
At an oxygen content of 41 vol %, the conversion of methane with φ = 3.0 makes it possible to obtain a gas with a hydrogen and carbon monoxide content of 44.3 and 21.4 vol %, respectively; the combustion temperature is 1021 K. The maximum yields of hydrogen and carbon monoxide are 1.58 and 0.87 mol, respectively. Thus, in the conversion of methane with an increased oxygen content for mixtures with the adiabatic combustion temperature not lower than 1000 K, the maximal output of hydrogen is less, and the yield of carbon monoxide is higher than with air conversion.
An increase in the initial temperature of the mixture leads to an increase in the adiabatic combustion temperature, concentration, and yield of carbon monoxide in the products. At the same time, the content and yield of hydrogen are reduced. An increase in the initial temperature of the mixture for every 100 K leads to an increase in the adiabatic temperature by approximately the same amount. Moreover, the relationship between the concentrations of the products and the initial temperature of the mixture is rather weak.
The thermodynamic calculation of the equilibrium parameters of the partial oxidation of natural gas makes it possible to obtain an upper estimate from the composition of the products and the temperature of the process.
REFERENCES
F. Chen, P. P. Zhang, Y. Zeng, et al., Appl. Catal. B: Environ. 279, 119382 (2020). https://doi.org/10.1016/j.apcatb.2020.119382
P. Bodhankar, S. Patnaik, and G. R. Kale, Int. J. Energ. Res. (2020). https://doi.org/10.1002/er.6283
P. Ebrahimi, A. Kumar, and M. Khraisheh, Emerg. Mater. 2020, 881 (2020). https://doi.org/10.1007/s42247-020-00116-y
N. Gao, M. Sliz, C. Quan, A. Bieniek, and A. Magdziarz, Renew. Energy 167, 652 (2021). https://doi.org/10.1016/j.renene.2020.11.134
N. M. Rubtsov, A. N. Vinogradov, A. P. Kalinin, A. I. Rodionov, I. D. Rodionov, K. Ya. Troshin, G. I. Tsvetkov, and V. I. Chernysh, Russ. J. Phys. Chem. B 13, 305 (2019). https://doi.org/10.1134/S1990793119020210
X. Tu, H. J. Gallon, M. V. Twigg, P. A. Gorry, and J. C. Whitehead, J. Phys. D: Appl. Phys. 44, 274007 (2011). https://doi.org/10.1088/0022-3727/44/27/274007
C. Wang, B. Wang, and S. Liu, J. Zou, Chem. Sel. 5, 13781 (2020). https://doi.org/10.1002/slct.202002890
N. N. Buravtsev, Yu. A. Kolbanovskii, I. V. Rossikhin, and I. V. Bilera, Russ. J. Phys. Chem. B 13, 273 (2019). https://doi.org/10.1134/S1990793119020027
S. Timmerberg, M. Kaltschmitt, and M. Finkbeiner, Energy Convers. Manage.: X 7, 100043 (2020). https://doi.org/10.1016/j.ecmx.2020.100043
A. Yu. Zaichenko, S. V. Glazov, E. A. Salgansky, V. M. Kislov, D. N. Podlesniy, A. I. Zhavoronkov, and M. V. Salganskaya, Theor. Found. Chem. Eng. 51, 673 (2017). https://doi.org/10.1134/S0040579517050396
A. M. Tereza, G. L. Agafonov, E. K. Anderzhanov, S. P. Medvedev, S. V. Khomik, S. K. Petrov, and M. V. Chernyshov, Russ. J. Phys. Chem. B 14, 654 (2020). https://doi.org/10.1134/S1990793120040247
V. M. Kislov, E. A. Salganskii, M. V. Tsvetkov, and Yu. Yu. Tsvetkova, Russ. J. Appl. Chem. 90, 716 (2017).https://doi.org/10.1134/S1070427217050081
S. B. Haghi, G. Salehi, M. T. Azad, et al., Pet. Chem. 60, 1251 (2020). https://doi.org/10.1134/S0965544120110109
A. Nikitin, A. Ozersky, V. Savchenko, et al., Chem. Eng. J. 377, 120883 (2019). https://doi.org/10.1016/j.cej.2019.01.162
H. Nourbakhsh, J. R. Shahrouzi, A. Zamaniyan, H. Ebrahimi, and M. R. J. Nasr, Int. J. Hydrogen Energy 43, 15703 (2018). https://doi.org/10.1016/j.ijhydene.2018.06.134
F. F. Tabrizi, S. A. H. S. Mousavi, and H. Atashi, Energy Convers. Manage. 103, 1065 (2015). https://doi.org/10.1016/j.enconman.2015.07.005
A. Lutz, R. W. Bradshaw, J. O. Keller, and D. E. Witmer, Int. J. Hydrogen Energy 28, 163 (2002). https://doi.org/10.1016/S0360-3199(02)00053-8
A. Antzara, E. Heracleous, D. B. Bukur, and A. A. Lemonidou, Energy Proc. 63, 6576 (2014). https://doi.org/10.1016/j.egypro.2014.11.694
D. Saebea, S. Authayanun, Y. Patcharavorachot, and A. Arpornwichanop, Energy Proc. 61, 2254 (2014). https://doi.org/10.1016/j.egypro.2014.12.120
W. H. Chen, M. R. Lin, J. J. Lu, Y. Chao, and T. S. Leu, Int. J. Hydrogen Energy 35, 11787 (2010). https://doi.org/10.1016/j.ijhydene.2010.08.126
Yu. M. Dmitrenko and P. A. Klevan, J. Eng. Phys. Thermophys. 84, 1296 (2011). https://doi.org/10.1007/s10891-011-0597-2
F. Guerrero, L. Espinoza, N. Ripoll, et al., Front. Chem. 8, 145 (2020). https://doi.org/10.3389/fchem.2020.00145
G. Ruiz, N. Ripoll, N. Fedorova, et al., Int. J. Heat Mass Transfer 136, 383 (2019). https://doi.org/10.1016/j.ijheatmasstransfer.2019.03.009
R. Horn, K. A. Williams, N. J. Degenstein, and L. D. Schmidt, J. Catal. 242, 92 (2006). https://doi.org/10.1016/j.jcat.2006.05.008
K. Otsuka, Y. Wang, E. Sunada, and I. Yamanaka, J. Catal. 175, 152 (1998). https://doi.org/10.1006/jcat.1998.1985
S. S. Kostenko, A. N. Ivanova, A. A. Karnaukh, and E. V. Polianczyk, Chem. Eng. J. 238, 100 (2014). https://doi.org/10.1016/j.cej.2013.09.012
B. G. Trusov, in Proceedings of the 14th International Conference on Chemical Thermodynamics (NIIKh SPbGU, St. Petersburg, 2002), p. 483.
V. V. Petrov, Yu. N. Varzarev, A. P. Starnikova, and Kh. A. Abdullin, Russ. J. Phys. Chem. B 14, 117 (2020). https://doi.org/10.1134/S199079312001025X
A. M. Tereza and E. K. Anderzhanov, Russ. J. Phys. Chem. B 13, 626 (2019). https://doi.org/10.1134/S1990793119040262
A. M. Tereza, S. P. Medvedev, and V. N. Smirnov, Acta Astronaut. 176, 653 (2020). https://doi.org/10.1016/j.actaastro.2020.03.045
V. M. Kislov, S. V. Glazov, E. A. Salgansky, A. F. Zholudev, and M. V. Salganskaya, Combust. Explos., Shock Waves 50, 518 (2014).https://doi.org/10.1134/S0010508214050037
E. A. Salgansky, A. Y. Zaichenko, D. N. Podlesniy, et al., Int. J. Hydrogen Energy 45, 17270 (2020). https://doi.org/10.1016/j.ijhydene.2020.04.177
M. A. Mujeebu, Appl. Energy 173, 210 (2016). https://doi.org/10.1016/j.apenergy.2016.04.018
Funding
This study was carried out as part of state assignment no. АААА-А19-119022690098-3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salgansky, E.A., Tsvetkov, M.V., Zaichenko, A.Y. et al. Thermodynamic Evaluation of Noncatalytic Conversion of Natural Gas with the Production of Synthesis Gas. Russ. J. Phys. Chem. B 15, 969–976 (2021). https://doi.org/10.1134/S1990793121060087
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793121060087