Abstract
At the wavelength λ = 253.7 nm, the photolysis of a C2H2F2Br2 mixture with oxygen was carried out at pressures of the latter ranging from 1 to 3.5 Torr. It is shown that under these conditions, upon the decay of one C2H2F2Br2 molecule, only one bromine atom is formed. At wavelengths 230, 240, and 250 nm, the absorption cross sections of one of the photolysis products, the C2F2BrO2 radical, are determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Earlier, in [1], we studied the photolysis of C2H2F2Br2 in the pressure range from 4 to 30 Torr in a mixture with oxygen at pressures of the latter ranging from 12 to 500 Torr. Interest in this hydrocarbon containing bromine atoms is caused by the fact that it is a hydrogen-containing analog of C2F4Br2 (halon 2402). The latter is still used to extinguish particularly complex fires in a number of countries, despite the high values of its ozone depletion potential (ODP = 8) and global warming potential (GWP = 1860) [2]. The reaction rate constant C2H2F2Br2 with the OH radical does not exceed 1.5 × 10–16 cm3 molecule–1 s–1 [3], which gives a value for the lifetime of about 200 years.
This study is a continuation of the study of the photolysis of C2H2F2Br2. The advantage of this substance over C2F4Br2 is that its ODP is 0.7–1.5 [2]. Such a low ODP value is explained by the fact that it contains hydrogen atoms, which ensure its destruction in the troposphere in reaction with the OH radical. The rate constant of this reaction at a temperature of 298 K is 2.46 × 10–4 cm3 molecule–1 s –1 [4] which gives the lifetime of C2H2F2Br2 in the atmosphere that is two orders of magnitude shorter than for C2F4Br2.
In contrast to [1], this study was carried out at oxygen pressures not exceeding 3.5 Torr, which made it possible to obtain additional results.
EXPERIMENTAL
The C2H2F2Br2 was mixed with O2 in a vacuum installation made of molybdenum glass with the shut-off valves made of glass and Teflon parts. Oxygen of a pure grade was stored in a glass flask, and liquid C2H2F2Br2 in ampules, isolated from light.
The optical setup consisted of a quartz cell 4 cm in diameter and 10 cm long and a low-pressure mercury lamp with a power of 8 W, which served as a radiation source with λ = 253.7 nm. To record the absorption spectra of the investigated substances in the ultraviolet and visible regions, a Specord M-40 spectrophotometer (Carl Zeiss, Germany) was used. The kinetics of photolysis C2H2F2Br2 was studied by measuring the optical density of the mixture at different times of irradiation in the absorption region of the starting substance and at a wavelength of 416 nm, corresponding to the absorption maximum of molecular bromine.
To determine the lamp intensity (I) under the given irradiation conditions, experiments were carried out with HBr, for which the quantum yield of photolysis (φ) upon irradiation with light with λ = 253.7 nm for both hydrogen atoms and bromine atoms was unity. The intensity of the lamp was found to be (1.9 ± 0.2) × 1015 quanta/(cm2 s). The setup for studying the photolysis of freons and the experimental procedure are described in more detail in [5].
Earlier, to interpret the experimental data obtained by us during the photolysis of C2H2F2Br2 in a mixture with oxygen at oxygen pressures exceeding 12 Torr, we proposed the following photolysis scheme of C2H2F2Br2 at a wavelength of 253.7nm [1]:
The method of semistationary concentrations, developed by Academician N.N. Semenov [6], allows us to obtain the following expression for the formation rate of molecular bromine:
where α = kII[O2]/(kI + kII[O2]) and σd is the dissociation cross section.
We have previously shown [1] that at sufficiently high oxygen pressures the quantum yield of the photolysis of C2H2F2Br2 is equal to unity; i.e., the formation of the second bromine atom in reaction (I) does not occur. In this study, we carried out photolysis at oxygen pressures in the range of 1 to 3.5 Torr on the assumption that, possibly, under these conditions, reaction (I) will compete with reaction (II).
It was determined in [1] that the ratio of constants kIV/kIII is 2.6; thus, expression (1) can be represented as follows:
where ß = (2.6 [O2]/[Br2] + 1)–1.
With an irradiation time of 1800 s, expression (2) will have the following form:
Figure 1 shows the dependence d [Br2] on (2.6[O2]/[Br2] + 1)–1 at the pressure of C2H2F2Br of 20 Torr and an irradiation time of 1800 s. From the tangent of the slope of this graph, the coefficient α = kII[O2]/(kI + kII[O2]). It was equal to 0.98 ± 0.3.
We also carried out photolysis of a mixture of oxygen at 1 Torr and C2H2F2Br2 at 18 Torr. Our kinetic curves of changes in the optical density at wavelengths ranging from 230 to 245 nm had an unusual shape (see Figs. 2a, 2b).
We assumed that such a change in optical density is due to the fact that the absorption cross section of the radical \({{{\text{C}}}_{{\text{2}}}}{{{\text{H}}}_{{\text{2}}}}{{{\text{F}}}_{{\text{2}}}}{\text{BrO}}_{2}^{ \bullet },\) formed in reaction (IV), significantly exceeds the absorption cross section of the starting substance. At the inflection points on these graphs, the optical density due to the presence of the original substance in the optical cell (DR, where R = C2H2F2Br2) becomes equal to the optical density due to the peroxide radical formed in reaction (IV) (DR', where R' = C2H2F2BrO2).
The Beer–Lambert law allows us to obtain the following expression:
where σR, R' is the absorption cross section at the corresponding wavelength.
Knowing the concentrations of freon and peroxide radical at the inflection points of the kinetic curves presented in Figs. 2a and 2b, we can find the ratio of the absorption cross sections σR'/σR in this wavelength range.
We have shown above that at low oxygen pressures, as well as at pressures above 12 Torr [1], reaction (I) can be excluded from the photolysis scheme. In this case, the change in the concentrations of the starting substance and photolysis products depending on the irradiation time is described by the following system of differential equations:
Using the method of semistationary concentrations, developed by Academician Semenov [6], we obtained the following expression for the concentration of the radical \({{{\text{C}}}_{{\text{2}}}}{{{\text{H}}}_{{\text{2}}}}{{{\text{F}}}_{{\text{2}}}}{\text{BrO}}_{2}^{\cdot },\) allowing us to calculate this value at each moment of irradiation:
Ratio k3/k4 was determined to be 0.38 in [1]. The concentration of molecular bromine was determined from the optical density at a wavelength of 416 nm. The dependence of this value on the exposure time is shown in Fig. 3.
The data obtained during the photolysis of a mixture C2H2F2Br2 at 18 Torr and oxygen at 1 Torr at the time instant corresponding to the inflection point (tinfl) of the curves in Figs. 2a and 2b are summarized in Table 1. The partial pressure of molecular bromine is taken from the graph in Fig. 3, the partial pressure of the consumed C2H2F2Br2 was considered to be double the pressure of the formed molecular bromine. The partial pressure \({{{\text{C}}}_{{\text{2}}}}{{{\text{H}}}_{{\text{2}}}}{{{\text{F}}}_{{\text{2}}}}{\text{BrO}}_{2}^{\cdot }\) was calculated by formula (5); and the absorption cross section of the C2H2F2BrO2 radical, according to formula (4).
We did not find any published data on the absorption cross sections of the C2H2F2BrO2 radical in the ultraviolet region of spectrum [7] with which we could compare our values. Therefore, we checked the correctness of our approach by investigating the dependence of the optical density of a mixture of bromomethane (CH3Br) with oxygen at a wavelength of 240 nm from the time of irradiation with light with a wavelength of 257.3 nm. Bromomethane was chosen because for this substance and for the radical \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}_{2}^{\cdot },\) formed during its photolysis, the absorption cross sections were reliably measured in the wavelength range of 190 to 300 nm [8].
Photolysis CH3Br can be represented by the following equations:
The following system of differential equations can be written for the photolysis scheme given above:
Based on this system of differential equations, one can calculate the concentration of the peroxide radical \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}_{2}^{\cdot }{\text{:}}\)
This expression can be converted to the form
The ratio kVI/kVII was determined by us earlier to be 26 [5], and the concentration of Br2 was determined from the graph of the dependence of the optical density at a wavelength of 416 nm on the time of irradiation.
The graph of the dependence of the optical density at a wavelength of 240 nm, D240, from the time of irradiation at the initial pressure of CH3Br equal to 40 Torr and oxygen pressure equal to 2.4 Torr is shown in Fig. 4. The optical density D240 before the start of irradiation corresponded to the initial pressure of CH3Br equal to 40 Torr. Irradiation of the mixture led to the consumption of CH3Br and the formation of molecular bromine. At the beginning of exposure, D240 decreased. At an irradiation time of 30 min, the decrease in optical density ceased, and then it increased. This was due to the fact that the radical \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}_{2}^{\cdot },\) formed in stage (VII), had absorption cross sections in the wavelength range of 220 to 250 nm, significantly exceeding the absorption cross sections of the initial CH3Br [7]. Therefore, a decrease in optical density at these wavelengths due to the consumption of CH3Br was compensated by its increase due to the accumulation of the radical \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}_{2}^{\cdot }.\)
The partial pressure of Br2calculated from the optical density at a wavelength of 416 nm was (0.25 ± 0.01) Torr. This meant that (0.5 ± 0.02) Torr of CH3Br was consumed, i.e., (39.5 ± 0.04) Torr of the initial freon, and the partial pressure \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}_{2}^{\cdot },\) formed by this time in reaction (VII), calculated by formula (7), was (0.41 ± 0.02) Torr.
At the time moment of 30 min, the optical density due to the presence of 39.5 Torr in the CH3Br cuvette became equal to the optical density due to the formed \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}_{2}^{\cdot }\) (0.41 Torr). This meant that
This value is in close agreement with the ratio \({{{{\sigma }_{{{\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}_{2}^{\cdot }}}}} \mathord{\left/ {\vphantom {{{{\sigma }_{{{\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}_{2}^{\cdot }}}}} {{{\sigma }_{{{\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{Br}}}}}}}} \right. \kern-0em} {{{\sigma }_{{{\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{Br}}}}}}}\) at a wavelength of 240 nm, equal to 96, which can be calculated from the values of these and the cross sections for the wavelength given in [7]. It seems to us that such a close coincidence of the \({{{{\sigma }_{{{\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}_{2}^{\cdot }}}}} \mathord{\left/ {\vphantom {{{{\sigma }_{{{\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}_{2}^{\cdot }}}}} {{{\sigma }_{{{\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{Br}}}}}}}} \right. \kern-0em} {{{\sigma }_{{{\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{Br}}}}}}},\) value obtained by us, with the highly reliable data given in [7], is evidence of the validity of using this approach in the case of the photolysis of C2H2F2Br2.
RESULTS AND DISCUSSION
In this study, the photolysis of C2H2F2Br2 was carried out by us in oxygen’s partial pressures ranging from 1 to 3.5 Torr. This range of oxygen pressures corresponds to the atmospheric pressure of the latter at altitudes 23 to 31 km above sea level, i.e., in those regions of the stratosphere in which the ozone concentration is close to the maximum [9, p. 146]. At these altitudes, the contribution of the bromine cycle to the destruction of the ozone layer ranges from 10 to 25% [9, p. 124]. The composition of the C2H2F2Br2 molecule includes two hydrogen atoms; i.e., such compounds that contain hydrogen atoms in their composition are considered as substitutes of CF3Br and C2F4Br2 when extinguishing fires [10].
The photolysis of C2F4Br2 (this substance is still used to eliminate fires [11] despite its danger to the ozone layer [12]) has been studied both theoretically [13] and experimentally [14, 15], while we did not find any published data on the photolysis or pyrolysis of C2H2F2Br2. Recently, the question of the use of hydrocarbons containing bromine atoms (freons) and hydrogen atoms for the elimination of fires has been intensively studied. Their significant disadvantage is their higher flammability compared to freons that do not contain hydrogen atoms. Therefore, for these substances, special methods of extinguishing fires are being developed. For example, in [16] it was proposed to throw containers containing halons and inert diluents directly into the fire zone.
Flames are extinguished by freons due to the chains of the chemical combustion reaction breaking. This allows the use of a small amount of freon, thereby reducing the amount of poisonous products of its decomposition.
In contrast to the quantum yield of bromine atoms during the photolysis of C2F4Br2 exceeding unity even when irradiated with light with a wavelength of 266 nm [15], the quantum yield of bromine atoms during the photolysis of C2H2F2Br2 at a wavelength of 253.7 nm at oxygen pressures of 12 Torr and higher did not exceed unity [1]. In this study, photolysis was carried out at oxygen pressures ranging from 1 to 3.5 Torr. One could expect, at low oxygen pressures, the decomposition reaction of the excited C2H2F2Br•* radical, leading to the formation of an additional bromine atom, to compete with the process of its deactivation on oxygen molecules. However, even at these pressures, the quantum yield of bromine atoms did not exceed unity. This is an additional benefit of freon C2H2F2Br2 over freon C2F4Br2 when these substances are used to extinguish a fire. In addition, the decrease in the quantum yield of bromine atoms is an important factor for the preservation of the ozone layer, since it is known that the length of the chains of the bromine cycle of ozone destruction in the lower stratosphere is tens of times longer than the length of the chains with the participation of chlorine atoms [17].
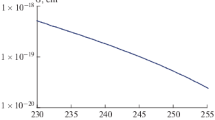
It can be assumed that the decrease in the quantum yield of bromine atoms during the photolysis of C2H2F2Br2 compared with C2F4Br2 is explained by the fact that, in accordance with [18, 19], the strength of the C–Br bond increases upon replacement of the halogen atoms in a hydrocarbon molecule containing bromine atoms by hydrogen atoms. In this study, we also determined the radical absorption cross sections \({{{\text{C}}}_{{\text{2}}}}{{{\text{H}}}_{{\text{2}}}}{{{\text{F}}}_{{\text{2}}}}{\text{BrO}}_{2}^{\cdot }\) at wavelengths 231, 235, 240, and 245 nm.
Over the past thirty years, the formation of peroxide radicals as a result of the photolysis or pyrolysis of various organic compounds and subsequent reactions of these radicals with molecular oxygen has been intensively studied [20–22]. These radicals play a huge role in atmospheric chemistry, participating in the formation and destruction of various pollutants in the troposphere. They also affect the processes leading to climate change and the lifetimes of compounds that deplete the ozone layer [23].
As for peroxide radicals containing bromine atoms, we found in the literature only one work devoted to the study of such reactions [24]. The absorption cross sections of peroxide radicals containing bromine atoms in the wavelength range 230–245 nm were measured by us, apparently, for the first time.
If C2H2F2Br2 is used to extinguish fires, then the pyrolysis of this compound, as well as photolysis, will lead to the formation of bromine atoms, C2H2F2Br• radicals, and the product of the interaction of the latter with the oxygen of the peroxide radical \({{{\text{C}}}_{{\text{2}}}}{{{\text{H}}}_{{\text{2}}}}{{{\text{F}}}_{{\text{2}}}}{\text{BrO}}_{2}^{\cdot }.\) The part of the C2H2F2Br2 molecules that can reach the lower stratosphere will decay under the influence of UV radiation, which will also lead to the formation of \({{{\text{C}}}_{{\text{2}}}}{{{\text{H}}}_{{\text{2}}}}{{{\text{F}}}_{{\text{2}}}}{\text{BrO}}_{2}^{\cdot }.\)
We note that the absorption cross sections of this radical determined by us in the wavelength range of 231 to 245 nm are several times higher than the absorption cross sections of ozone at these wavelengths [25].
CONCLUSIONS
1. It is shown that at oxygen pressures ranging from 1 to 3.5 Torr, the rate constant of the decomposition reaction of an excited C2H2F2Br•* radical with the formation of a bromine atom is negligible compared to the rate constant of its deactivation on oxygen molecules.
2. It was concluded that at these pressures, as well as at oxygen pressures of 12 Torr and higher, the formation of the second bromine atom in the decomposition of the C2H2F2Br•* radical does not occur, and the quantum yield of the photolysis of C2H2F2Br2 is unity.
3. The cross sections for the absorption of the radical \({{{\text{C}}}_{{\text{2}}}}{{{\text{H}}}_{{\text{2}}}}{{{\text{F}}}_{{\text{2}}}}{\text{BrO}}_{2}^{ \bullet }\) are calculated at wavelengths of 231 to 245 nm using the coordinates of the inflection points on the graphs of changes in the optical density of the C2H2F2Br2 mixture with oxygen upon irradiation of this mixture with light with a wavelength of 253.7 nm.
4. In a similar way, using the coordinates of the inflection point on the graph of the changes in the optical density of the CH3Br mixture with oxygen upon irradiation of this mixture (λ = 253.7 nm), the ratio of the radical absorption cross section \({\text{C}}{{{\text{H}}}_{{\text{3}}}}{\text{O}}_{2}^{\cdot }\) to the absorption cross section of CH3Br at a wavelength of 240 nm is calculated. The result obtained is in excellent agreement with the published data.
REFERENCES
I. K. Larin, T. I. Belyakova, N. A. Messineva, A. I. Spasskii, and E. M. Trofimova, Russ. J. Phys. Chem. B 14, 893 (2020).
Halocarbons: Ozone Depletion and Global Warming Overview (NASA, 2006).
J. B. Burkholder, R. R. Wilson, T. Gierczak, et al., J. Geophys. Res. 96, 5025 (1991).
C. Chiorboli, R. Piazza, M. L. Tosato, and V. Carassiti, Coord. Chem. Rev. 125, 241 (1993).
T. I. Belyakova, I. K. Larin, N. A. Messineva, and E. M. Trofimova, Russ. J. Phys. Chem. B 12, 352 (2018).
N. N. Semenov, Chain Reactions (Nauka, Moscow, 1986; United Sci. Tech. Press, 1934).
Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies (JPL Publ., Pasadena, CA, 2015), No. 19-5.
Chemical Kinetics and Photochemical Data for Use in Stratospheric Modeling, Evaluation No. 15 (California Inst. Technol., Pasadena, CA, 2006).
I. K. Larin, Chemical Physics of the Ozone Layer (GEOS, Moscow, 2018) [in Russian].
T. Noto, V. Babushok, A. Hamins, and W. Tsang, Combust. Flame 112, 147 (1998).
Yu. N. Shebeko, in Jubilee Collection of Works of VNIIPO (VNIIPO, Moscow, 1997), p. 69 [in Russian].
Montreal Protocol on Substances that Deplete the Ozone Layer, United Nations Environment Programme (Halons Tech. Options Committee, Monreal, Canada, 2006).
Aqeel A-Hussein and Abbas A-Ali Drea, Basrah J. Sci. C 30, 132 (2012).
I. K. Larin, T. I. Belyakova, N. A. Messineva, A. I. Spasskii, and E. M. Trofimova, Kinet. Catal. 52, 513 (2011).
P. Zou, W. S. McGivern, O. Sokhabi, A. G. Suits, and S. W. North, J. Chem. Phys. 113, 7149 (2000).
A. M. Tsarev and D. A. Zhuikov, Izv. Samar. Nauch. Tsentra RAN, Mekh. Mashinostr. 9, 777 (2007).
I. K. Larin, Russ. J. Phys. Chem. B 13, 548 (2019).
Luom Yu-Ran, Handbook of Bond Dissociation Energies in Organic Compounds (CRC, Boca Raton, FL, 2002), p. 158.
M. Szwarc and A. H. Sehon, J. Chem. Phys. 19, 656 (1951).
I. K. Larin, Russ. J. Phys. Chem. B 11, 189 (2017).
O. J. Nielsen, M. S. Johnson, T. J. Wallington, and L. K. Christensen, Int. J. Chem. Kinet. 34, 283 (2002).
J. J. Orlando and G. S. Tyndalla, Chem. Soc. Rev. 41, 6294 (2012).
G. S. Tyndall, R. A. Cox, C. Granier, et al., J. Geophys. Res. 106, 12157 (2001).
E. Villenave, S. Moisan, and R. Lesclaux, J. Phys. Chem. A 107, 2470 (2003).
I. K. Larin, Chemical Physics of the Ozone Layer (GEOS, Moscow, 2018), p. 39 [in Russian].
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Larin, I.K., Belyakova, T.I., Messineva, N.A. et al. Photolysis of C2H2F2Br2 Mixture with O2 in the Oxygen Pressure Range 1–3.5 Torr. Russ. J. Phys. Chem. B 15, 795–800 (2021). https://doi.org/10.1134/S1990793121050195
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793121050195