Abstract
The prospects of using catalysts based on immobilized lipase in the transesterification of triglyceride of oleic acid (TOA) with methanol in supercritical (SC) carbon dioxide are shown. At the optimum temperature of 40°C, CO2 pressure of 15.0 MPa, and a TOA : methanol molar ratio of 1 : 3, the yield of oleic acid methyl ester is significantly higher than that obtained in methanol at atmospheric pressure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Currently, special attention is being paid to alternative methods of fuel recovery from renewable vegetable raw materials [1–4]. The process for the preparation of esters of fatty acids (EFAs) (biodiesel) by the transesterification reaction of vegetable oils (triglycerides of fatty acids (TFAs)) with lower alcohols (methanol or ethanol) [5] by the reaction
has been widely applied.
It is possible to carry out transesterification of TFAs with lower alcohols in addition to homogeneous [1, 2] acidic or basic catalysts, which ensure the process of transesterification under mild conditions (30–65°C) at a high speed. In the case of heterogeneous catalysts such as alkaline earth metal oxides, zirconium oxide, zeolites, and others, the process is carried out under more severe conditions (up to 200°C) [1, 2, 6]. The difficulty of separating and purifying the product from the catalyst is a disadvantage of homogenous and heterogeneous processes [1, 2, 6].
It is proposed to carry out the transesterification of TFAs in the presence of an enzyme-lipase of microbiological, plant, and animal origin (lipoprotein lipase, pancreatic lipase, endotepialny lipase, etc. [2, 6, 7].
It is noted in the literature that heterogeneous catalysts reduce activity in the presence of water, and alkalis are sensitive to the presence of water and free fatty acids in the raw materials. Moreover, it is known that enzymes are able to maintain activity and exhibit high selectivity in the presence of water in the raw material [6]. Nevertheless, work is underway to create sustainable biocatalysts based on lipase immobilized on various carriers [8, 9].
The current focus is on the development of noncatalytic processes for the transesterification of TFAs by lower alcohols under sub- and supercritical (SC) conditions [10–16]. Lower alcohols such as methanol and ethanol can mix only in a limited way with TFAs in normal conditions due to the high polarity and the presence of hydrogen bonds. When carried out under SC conditions (critical points: 512.6 K and 8.09 MPa for methanol and 513.9 K and 6.14 MPa for ethanol), the solubility of triglycerides increases and they form homogeneous systems with alcohols. By optimizing the conditions of the process (pressure, temperature, solvent composition, solvent-to-feed ratio, etc.), a yield above 90% of (EFAs) can be achieved. SC-CO2, which is actively used to extract TFAs from plant raw materials [6, 17], has been used in transesterification only as a cosolvent of SC lower alcohols [6, 18].
This work presents the results of the studying the biocatalytic transesterification of triglyceride of oleic acid (TOA) with methanol in an SC-CO2 medium. Both native lipase and lipase immobilized on magnetic particles of Fe3O4 were used to carry out the process. The magnetic particles chosen as the enzyme carrier are selected based on the ease of separation from the reaction product.
EXPERIMENTAL
The following reagents and solvents are used in the study: iron chloride (II) six-state FeCI3 ⋅ 6H2O (pure, Reachem); iron sulfate (II) seven-water Fe2(SO4)3 ⋅ 7H2O(Reachem); phosphate buffer of composition Na2HPO4 ⋅ 2H2O + KH2PO4 (pH 6.86, Uralkhiminvest); NaOH sodium hydroxide, 98% (analytically pure, Neva-reactiv); methanol(С, Reachem); ethanol(chemically pure, Reachem); TOA (99%, Sigma-Aldrich); serum albumin standardized lipase L3126 Type II (100–400 un./mg, Sigma-Aldrich); 3-aminopropiltrietoksisilan NH2(CH2)3Si(OC2H5)3 (98%, Sigma-Aldrich); glutaric dialdehyde(25%, Fluka); diphenylamine C6H5)2NH (analytically pure, Neva-reactiv); and carbon dioxide(99.8%, Tver-gazservis).
To prepare magnetic nanoparticles (MNPs), an aqueous solution (25 mL) of a mixture of iron salts in equimolar amount (2.8 g FeSO4 ⋅ 7H2O and 2.7 g FeCI3 ⋅ 6H2O) was added dropwise to the NaOH solution (1.5 M, 250 mL) with constant stirring. The resulting Fe3O4 black precipitate was separated from the reaction medium with a neodymium magnet, washed with water to a neutral pH, and then placed in 50 mL of 95% ethanol. Next, 0.3 mL of a solution of 3-(aminopropyl)-triethoxysilane was added to the ethanol suspension of the resulting 2 g MNPs to modify their surface with amino groups, stirred for 7 h, then washed to a neutral pH. For enzyme covalent crosslinking (formation of an azomethine bond on the surface of the biocatalyst), 25 mL of a 1% glutaric dialdehyde solution was added to the modified MNPs, stirred for 2 h, and then washed with a five-fold excess of distilled water. The resulting modified MNPs were stirred for 6 h with a 50 mL lipase buffer solution (1 g lipase per 50 mL phosphate buffer). All operations to separate the MNPs of the lipase biocatalyst/Fe3O4 from the solution were carried out with a neodymium magnet.
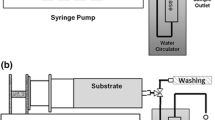
A Parr Instruments 4307 high-pressure reactor (United States) with the total flask volume of 250 cm3 and the maximum working pressure of 60 MPa was used (Fig. 1) to obtain oleic acid methyl ester (OAME) in an SC-СО2 medium. Plunger pump Supercritical 24 Pump CP (SSI, United States) was used to pump the carbonic acid. The standard experiment was conducted as follows.
A charge of the biocatalyst (1.0 g of lipase/Fe3O4 or 0.5 g of free lipase), 50 mL of TOA (density 0.915 g/mL), and 6.3 mL of methanol (molar ratio TOA : methanol = 1 : 3) were added to the Teflon flask of the reactor. The reactor was purged thrice with carbon dioxide at 20 MPa; after the pressure was stabilized, more liquid CO2 was pumped until the reactor was filled. The reactor was heated to a predetermined temperature and the reaction was started. The test was carried out for 3 h. The CO2 pressure ranged from 10 to 30 MPa.
Comparative tests for the transesterification of TOA were carried out in the same reactor at the atmospheric pressure of nitrogen in the temperature range 30–60°C with the same molar ratio of TOA to methanol (1 : 3).
The reaction mixture was analyzed by gas chromatography with the mass spectroscopic detection of substances on a GS-2010 gas chromatograph (Shimadzu, Japan) equipped with a 30 m × 0.25 mm × 0.25 μm HP-1MS capillary column and a GSMS-QP 2010S quadrupole mass spectrometer (Shimadzu, Japan). The analysis was carried out in the thermoprogramming mode: exposure for 5 min at 80°C, linear heating from 80 to 105°C (10°C/min), linear heating from 105 to 250°C (25°C/min), and exposure at 250°C for 3 min. Ultrahigh purity helium 6.0 was used as the gas carrier (54.5 mL/min, linear speed in a cm column, 36 cm/s), and injector temperature 260°C. Diphenylamine was used as the internal standard.
The effectiveness of biocatalytic transesterification was estimated by the yield of OAME (Y, %) achieved in 180 minutes in the presence of a free and immobilized enzyme, the amount of which was calculated by the formula Y = 100Ci/Co, where Co is the theoretically achievable concentration of OAME in the reaction mixture and Ci is the practically achieved concentration of OAME in 180 min.
RESULTS AND DISCUSSION
Figure 2 shows the dependence of the OAME yield on temperature in the presence of different biocatalysts. The presented dependence shows that the effectiveness of native lipase during the transesterification of TOA in methanol at atmospheric pressure is higher (Y = 30%) than immobilized lipase (Y = 24%). When carried out in an SC-CO2 medium, on the contrary, immobilized lipase is more effective than native lipase (Y = 67 and 55%, respectively).
As shown in Fig. 3, the maximum yield in both native and immobilized lipase is achieved at CO2 15 MPa (55 and 67%, respectively). There was a decrease in the product yield with a further increase in the CO2 pressure.
Figure 4 shows a diagram of the maximum product yields during the transesterification of TOA in methanol (atmospheric pressure) and in an SC-CO2 medium. The presented data show that in the SC-CO2 medium the effectiveness of native and immobilized lipase is 1.8 and 2.8 times higher, respectively.
For both biocatalysts, the maximum efficiency is achieved at 40°C, which may be related to the inactivation of the enzyme at a higher temperature due to the subsequent denaturation.
In the presence of the lipase/Fe3O4 biocatalyst, the total conversion of the TOA in an SC-CO2 medium at 40°C and 15 MPa is achieved in 340 min, while in a methanol medium, at atmospheric pressure, this requires 610 min.
For immobilized enzymes, the stability of the lipase/Fe3O4 biocatalyst in seven consecutive TOA transesterification cycles in an SC-CO2 medium and methanol is of greater importance. The data obtained (see Fig. 5) show that this biocatalyst can be reused in both media. At the same time, its stability in SC-CO2 is higher than in methanol: after being used seven times in these media it loses 28 and 37% of its efficiency, respectively.
Thus, in an SC-CO2 medium, the lipase enzyme is more efficient in the transesterification reaction of TOA up to its methyl ester than in a methanol medium. The increase in the product’s yield may be due to the fact that a faster mass transfer takes place in the SC-CO2 medium. The use of Fe3O4 magnetic particles to immobilize lipase allows the biocatalyst to be separated easily from the product.
REFERENCES
N. G. Shemelis and M. M. Jorge, AIMS Energy 5, 425 (2017).
N. Saifuddin, A. Samiuddin, and P. Kumaran, Trends Appl. Sci. Res. 10, 1 (2015).
R. Gray, Biofuels Annual, Russian Federation (U. S. Dep. of Agriculture, 2016), p. 1.
A. Dhar and A. K. Agarwal, Fuel 119, 70 (2014).
R. Lokanatham and K. Ravindranath, Int. J. Eng. Res. Developm. 6, 35 (2013).
K. T. Lee, S. Lim, Y. L. Pang, H. C. Ong, and W. T. Chong, Prog. Energy Combust. Sci. 45, 54 (2014).
A. E. Rogozhin, Cand. Sci. (Chem.) Dissertation (Dzerzhinsk, 2017).
A. D. Q. Melo, F. F. M. Silva, J. C. S. dos Santos, R. Fernández-Lafuente, T. L. G. Lemos, and F. A. D. Filho, Molecules 22, 2165 (2017).
N. B. Carvalho, B. T. Vidal, A. S. Barbosa, M. M. Pereira, S. Mattedi, L. S. Freitas, A. S. Lima, and C. M. F. Soares, Int. J. Mol. Sci. 19, 1829 (2018).
K. G. Bogolitsyn, A. A. Krasikova, and I. A. Gusakova, Russ. J. Phys. Chem. B 10, 1048 (2016).
S. V. Mazanov, A. R. Gabitova, L. H. Miftahova, R. A. Usmanov, F. M. Gumerov, Z. I. Zaripov, V. A. Vasil’ev, and E. A. Karalyn, Russ. J. Phys. Chem. B 10, 1099 (2016).
V. I. Anikeev, D. A. Stepanov, and A. B. Ermakova, Russ. J. Phys. Chem. A 85, 1336 (2011).
H. J. Navarro-Díaz, S. L. Gonzalez, B. Irigaray, I. Vieitez, I. Jachmanián, H. Hense, and J. V. Oliveira, J. Supercrit. Fluids 93, 130 (2014).
K. Bunyakiat, S. Makmee, R. Sawangkeaw, and S. Ngamprasertsith, Energy Fuels 20, 812 (2006).
J. Cheng, T. Li, N. Peng, R. Huang, J. H. Zhou, and K. F. Cen, Fuel Proces. Technol. 131, 409 (2015).
D. Zhou, L. Qi, B. Q. Qiao, Q. Q. Xu, and J. Zh. Yin, J. Supercrit. Fluids 120, 395 (2017).
V. I. Bogdan, A. E. Koklin, V. G. Krasovsky, V. V. Lunin, Ya. E. Sergeeva, A. A. Ivashechkin, and E. P. Feofilova, Russ. J. Phys. Chem. B 8, 1004 (2014).
C. Bertoldi, C. da Silva, J. P. Bernardon, M. L. Corazza, L. C. Filho, J. V. Oliveira, and F. C. Corazza, Energy Fuels 23, 5165 (2009).
Funding
The study was carried as a part of projects financed by the Russian Foundation for Basic Research (grant nos. 18-29-06004 and 16-08-00158).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Drozdova
Rights and permissions
About this article
Cite this article
Lakina, N.V., Sulman, E.M., Doluda, V.Y. et al. Biocatalytic Transesterification of Oleic Acid Triglyceride in Supercritical Carbon Dioxide. Russ. J. Phys. Chem. B 14, 1077–1080 (2020). https://doi.org/10.1134/S1990793120070106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793120070106