Abstract
In this paper, we experimentally studied the limiting conditions for the combustion of hydrogen, methane, and propane in mixtures with air or oxygen at pressures below atmospheric. A significant effect is shown for the size of the reaction vessel and the ignition energy on both the minimal pressure value and the change in the concentration range of flame propagation. The dimensions of the reaction vessel and the magnitude of the ignition energy, the increase of which does not lead to a change in the minimal pressure and concentration region of flame propagation, are established. The minimal pressures Pmin of gas mixtures, below which flame propagation is impossible, were determined. A qualitative interpretation of the data is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The study of the limiting conditions for the combustion of gases at low pressures has been the subject of quite a large number of works [1–12]. A characteristic feature of combustion under these conditions is a narrowing of the concentration range of flame propagation with decreasing pressure until the lower and upper concentration limits close at the minimum pressure Pmin, below which mixtures with any content of fuel and oxidant are not able to propagate the flame. The change in the limiting pressure for combustion with a change in the concentration of the combustible gas or vapor can be basically described by a U-shaped curve. The analysis of the results of these works shows that the data for Pmin and the corresponding mixture compositions are contradictory.
The dependence of the concentration limits of flame propagation for acetone vapor was experimentally determined for various pressures [1]. The experiments were carried out in reaction vessels in the form of vertical tubes, with diameters of 6 and 7.5 cm. The Pmin value was found to be 13 kPa for combustion in air and 4 kPa for combustion of acetone vapors in an oxidizing environment that is a mixture of 50 vol % O2 and 50 vol % N2.
The spark ignition of CH4–air mixtures near the lower concentration limit of flame propagation was studied at low pressures [2]. The experiments were carried out in a glass reaction vessel with a diameter of 22 cm and a height of 36 cm (volume is 13.7 dm3). The spark source of ignition produced energy from 1 to 600 mJ, with a discharge duration from 20 to 200 μs. The initial pressure was 4–40 kPa. The minimal spark energy Emin required to ignite the mixture was found to substantially depend on the pressure. In this case, the Emin values in the specified pressure range are in the range of 1–1000 mJ, and the minimum energy increases significantly with decreasing pressure.
The behavior of the concentration limits of flame propagation in mixtures of hydrogen and formaldehyde vapors with air was studied in [3, 4]. The Pmin value for hydrogen was found to significantly depend on the diameter of the vertical reaction tube, which has a length of 960 mm. The Pmin values were 6.7, 7.9, and 15.8 kPa at tube diameters of 8, 16, and 25 mm, respectively. For formaldehyde, experiments were conducted in a pipe with a diameter of 40 mm and a height of 80 mm at 120°C, using spark discharge for the ignition of the mixtures. The Pmin value was 4.6 kPa at formaldehyde vapor concentrations from 30 to 70 vol %.
The concentration limits of flame propagation in H2–O2–N2 mixtures were measured at pressures of 5.3 × 10–2–2.6 kPa [5]. The experiments were carried out in two cylindrical reaction chambers with the following dimensions: with a diameter of 0.6 and 1.2 m and a height of 1.8 and 3.7 m, respectively. A spark ignition source with an energy of up to 2700 J was used. The concentration limits of flame propagation at a pressure of 2.6 kPa were found to be the same as those at atmospheric pressure. The value of Pmin when the mixture was ignited in a chamber with a diameter of 0.6 m with a spark with an energy of 200 J (the spark gap was 6.3 cm) was 5.7 × 10–2 kPa for an H2–O2–N2 mixture with an O2 : N2 ratio of 60 : 40. The same Pmin value was obtained in the chamber with a diameter of 1.2 m and ignition energy of 2700 J (the spark gap was 24 cm).
The limits of flame propagation at low pressures for ethane, propane, n-butane, and n-pentane in air were experimentally studied in [6]. The experiments were conducted in a vertical tube with a diameter of 4 cm and a height of 80 cm. The ignition was carried out by both a single electric spark and a series of sparks. In this case, different values for Pmin were obtained. For example, in the case of ethane, Pmin was 26 kPa for a single spark and 8.2 kPa for a series of sparks. For the propane–oxygen mixture, Pmin was 4 kPa. In general, for ignition by a series of sparks, the concentration range of flame propagation at low pressures is much wider than in the case of ignition by a single spark.
The concentration limits of flame propagation in propane–air mixtures were measured at 0.53–92 kPa [7, 8]. The experiments were carried out in a vertical reaction vessel with a diameter of 50 mm and a height of 150 cm. The mixture was ignited by a spark with a spark gap between the electrodes of 2 mm. At an increase in the spark energy to 2 J, the Pmin value was found to decrease and remains constant at a further increase in the energy to 8 J.
The ignition of methane–air mixtures using ignition sources at low pressures was studied in [9]. The experiments were performed in a 360 cm3-chamber using an electric spark and a plasma jet with energies of 120 mJ and 2.7 J, respectively, as ignition sources. At using a plasma jet, the concentration range of flame propagation was found to be much wider due mainly to rich mixtures. The Pmin value is about 2 kPa for a plasma jet and a spark with energy of 2.7 J and 7 kPa for a spark with energy of 120 mJ.
Experimental studies to determine the concentration limits of flame propagation and some other combustion characteristics for ethanol, cyclohexane, and isopropanol vapor were performed at low pressures (0.25–100 kPa) and elevated temperatures (from 20 to 150°C) [10]. The concentration limits were determined in setup by a reaction vessel with a diameter of 100 mm and a height of 900 mm. The mixture was ignited by an electric spark with a power of 10 W and a duration of 0.5 s. The fact the detachment of the flame front from ignition electrodes was used as a criterion for flame propagation.
An experimental determination of the concentration limits of flame propagation in gas–air mixtures (combustible gases are methane, carbon monoxide, and hydrogen) was carried out at low pressures (in the range from 0.5 to 100 kPa) [11]. Experiments were performed in reaction vessels with volumes of 53 and 100 dm3. Pmin values are determined for various parameters of the ignition source (arc discharge). Ignition energy was up to 30 J. The limiting pressure, at which flame propagation was found is possible, was found to be determined not by the size of the reaction vessel, but by the characteristics of the ignition source.
A decrease in the inhibiting ability of halogenated hydrocarbons with respect to an ethylene-air flame at a decrease in pressure from 100 to 17 kPa was shown in [12].
The concentration limits of flame propagation in hydrogen–oxygen mixtures were determined at temperatures from 77 to 290 K [13]. Experiments were performed in a reaction vessel in the form of a vertical tube with a diameter of 65 mm and a height of 450 mm. The ignition energy varied in the range from 1.5 to 36 J. In the range of hydrogen concentrations from 55 to 75 vol % and at 77 K, the minimal pressure at which flame propagation was possible was found to be 20–21 kPa.
The concentration limits of flame propagation in hydrogen-oxygen mixtures were determined at room temperature [14]. The experiments were carried out in a reaction vessel in the form of a vertical tube with a diameter of 54 mm and a height of 550 mm. The ignition energy ranged from 0.2 to 32 J. The minimal pressure, at which flame propagation is possible, was found to be 6.6 kPa. In the pressure range from 26 to 100 kPa, the concentration limits of flame propagation is shown not to depend on pressure.
From the above brief analysis, it is clear that conditions for the occurrence and propagation of the flame significantly change in the low-pressure region. In this case, the determining parameters are the size of the reaction vessel and the magnitude of the ignition energy. However, no published works known to the authors justified the choice of these parameters, which may raise doubts regarding the reliability of the obtained results.
This paper experimentally examines the limiting conditions for the combustion of hydrogen, methane, and propane in mixtures with air and oxygen at pressures below atmospheric in reaction vessels of various sizes at various values of the ignition energy.
EXPERIMENTAL
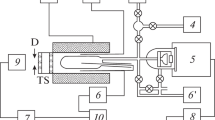
All reaction vessels excepting the smallest one had viewing Plexiglas windows. In experiments with reaction vessels of type a, b, and c, a mixer (volume is V = 0.025 m3) with mechanical mixing of gases was used for the preparation of gas mixtures, and a mixer (V = 0.5 m3) with convective mixing in vessel of type d was used (Fig. 1).
Gas mixtures were prepared by partial pressures in mixers pre-evacuated to a residual pressure of 13–26 Pa. The combustible component of the mixture was dosed using a manovacuometer. The reaction vessel was evacuated to the above pressures, and it was repeatedly (at least five times) purged with the prepared mixture. The composition of the initial mixtures was controlled by chromatography analyzing samples taken from the mixer. The discrepancy in the fuel concentrations in different samples did not exceed 0.15 vol %. The initial pressure in the reaction vessel was measured with a vacuum gauge (at operating pressures from 660 Pa and below) and a mercury manometer (at operating pressures above 660 Pa). In this case, the error in measuring pressure did not exceed 13 Pa for a values of 660 Pa and below or 133 Pa for values above 660 Pa. The mixture was ignited by burning a nichrome wire with a diameter of 0.0005 m and a length of 0.03 m in the lower part of the vessel. The voltage for burning the wire was applied from a capacitor bank. The magnitude of the measurement error of the ignition energy was 3.5 J for energy values of up to 60 and 10 J for larger energy values. The maximal pressure of the explosion in the vessel as a result of combustion was measured by a pressure gauge with a signal recorded by an oscilloscope. The absolute measurement error was 0.4%. The time constant of the gauge did not exceed 50 ms. Flame propagation to the upper part of the vessel was recorded visually and by tungsten-rhenium thermocouples with a diameter of 10 μm recorded on an oscilloscope, as well as by high-speed filming. The flame propagation to the top of the reaction vessel was taken as the flame propagation criterion for determining mixtures of limiting composition and minimal pressure Pmin.
RESULTS AND DISCUSSION
The results of studies of the limiting conditions for the combustion of hydrogen-air and hydrogen-oxygen mixtures in vessels with diameters from 0.3 to 2.5 m at ignition energy of 50 J are presented in Fig. 2. The curves limiting the concentration regions of flame propagation are U-shaped. Lowering the initial pressure results in the closure of the lower and upper concentration limits. The minimal pressure, at which flame propagation is possible, was 0.3 kPa for hydrogen–air mixtures and 0.076 kPa for hydrogen–oxygen mixtures in the studied reaction vessels.
Concentration region of flame propagation of hydrogen in air and in oxygen at pressures below atmospheric and ignition energy of 50 J: (1) air mixtures, (2) oxygen mixtures; (△) vessel b, (◻) vessel c, and (⚪) vessel d (see Fig. 1).
The Pmin values obtained by other authors for mixtures of hydrogen with air and oxygen in small reaction vessels, as a rule, were significantly higher than those measured in this study. For example, Pmin is 0.66 kPa for an oxyhydrogen mixture [13, 14].
Figure 3 shows the record of flame propagation along a hydrogen–air mixture in a reaction vessel with a diameter of 0.5 m at an initial pressure of 0.37 kPa. As it increases in size, the rising flame spot reaches the upper part of the reaction vessel. The diameter of the spot at the initial stage of combustion is about 0.11 m, which is significantly less than the diameter of the vessel. This suggests that the size of this reaction vessel is not critical for flame propagation.
A characteristic feature of combustion is that the ranges of pressures and concentrations in which the transition from a combustible mixture to a non-combustible one takes place are relatively small and are 0.04–0.05 kPa in pressure and less than 0.5 vol % by concentration. At the same time, for atmospheric and higher pressures, different authors [15–17] showed the existence of concentration zones from both limits, where incomplete combustion of the mixture was observed. Further, the flame in these mixtures can propagate only from the bottom up. These features are believed to be due to the effect of natural convection. In the low-pressure region, the role of natural convection becomes less significant. As shown in [18, 19], there are practically no external signs of natural convection already at 0.93 kPa (convective pop-up flame). However, visual observations and the record of the flame propagation (Fig. 3) indicate that a slight convection effect occurs at 0.25–0.30 kPa. However, at these and lower pressure values, natural convection loses its essential role in flame propagation unlike that observed at atmospheric pressure. Perhaps, the existence of narrow transition zones from a combustible to non-combustible mixture at low pressures is caused by this circumstance.
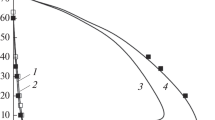
The experimental results for the determination of the concentration range of flame propagation and the minimal pressures of mixtures of methane and propane with air and with oxygen are presented in Figs. 4 and 5, respectively. The Pmin values were 0.706 and 0.093 kPa for mixtures of methane with air and oxygen and 0.666 and 0.08 kPa for similar mixtures of propane, respectively. The values of the minimal pressure for the methane–oxygen and the propane–oxygen mixtures slightly differ from Pmin for the hydrogen–oxygen mixture.
Concentration regions of flame propagation of methane in air and in oxygen at pressures Pini below atmospheric and ignition energy of 185 J: (1) mixtures of methane with air, (2) mixtures of methane with oxygen, (△) vessel b, and (⚪) vessel d (see Fig. 1).
Figure 6 presents experimental data on the minimal pressure for the hydrogen–air and hydrogen–oxygen mixtures for various energies E of the ignition source. As can be seen in the Fig. 6, an increase in the ignition energy leads to a decrease in Pmin for hydrogen–air mixtures that is 0.12 kPa at energy of 185 J. A further increase in energy (almost by an order of magnitude) does not cause a change in Pmin. At the same time, the minimal pressure for hydrogen–oxygen mixtures does not change, remaining constant at 0.076 kPa with increasing energy, starting from 50 J. As shown above, similar values of for Рmin were obtained in [5]. Pmin was 0.12 kPa for hydrogen–air mixtures and 0.06 kPa for hydrogen–oxygen mixtures. It should be noted that when the ignition energy is less than 50 J and is further reduced, Pmin for the hydrogen–oxygen mixture increases significantly, as shown in [20]. It can be assumed that the value of the ignition energy equal to 185 J for the hydrogen-air mixture and 50 J for the hydrogen–oxygen mixture is the limit, starting from which Pmin ceases to depend on E. The dependence of the minimal pressure on the magnitude of the ignition energy in the range from 20 to 200 J for hydrogen–air mixtures is satisfactorily described by the empirical equation:
Dependence of the minimal pressure Pmin on the ignition energy E for mixtures of hydrogen with air and with oxygen: (1) mixtures of hydrogen with air, (2) mixtures of hydrogen with oxygen, (⚪) this work, and (△) [5].
where Pmin is expressed in Pa, and E is in J.
The results of studies of the effect of ignition energy on the minimal pressure for air and oxygen mixtures of hydrocarbons are given in Table 1. As in the case of the hydrogen–oxygen mixture, the ignition energy of more than 50 J does not affect the minimal pressure of oxygen mixtures of methane and propane. For air mixtures of the studied hydrocarbons, an increase in E to 185 J leads to a decrease in Pmin. A further increase in energy does not cause a decrease in Pmin.
It is interesting to consider the question whether Pmin limited by the ignition energy is the fundamental characteristic of a combustible mixture and why the minimal pressure at increasing ignition energy E ceases to depend on the ignition energy at its high values in our experiments (again, under the conditions of our experiments). For this purpose, it is possible to analyze the conditions for ignition of a combustible mixture by an electric spark using the data of [21]. According to these data, for a spark to ignite a gas, the radius R of the volume in which temperature T exceeds the adiabatic flame temperature, must have an equal order of magnitude to the characteristic thickness δ of the flame front. The δ value is described by the following relation [21]:
where \(\ae \) is the coefficient of thermal diffusivity of the gas mixture, and Un is the normal combustion rate of this mixture.
The value \(æ \) ~ 1/p (where p is the mixture pressure), and the value of Un change relatively little with increasing p. Therefore, we can assume with a certain degree of approximation that, for decreasing pressure, the thickness of the flame front increases quite rapidly. At the same time, the value of R is relatively weakly dependent on spark energy E (R ~ E1/3) according to [21]. In fact, the dependence of R on E is apparently even weaker because the spark has a limited transverse size, which increases relatively weakly with increasing E. At the same time, the value of R at high ignition energies almost ceases to depend on E reaching some critical value Rcr.
Thus, the δ value with decreasing pressure increases up to the Rcr value. At further decreases in pressure, the δ value continues to increase while the characteristic size of the region occupied by the gas mixture heated by the spark remains constant, i.e., the condition of R ~ δ is violated. Therefore, increases in the spark energy within the limits used in this work does not lead to a decrease in Pmin, which causes the minimal pressure to be constant at high ignition energies. The value for Pmin certainly depends on δ, i.e., on the properties of the combustible mixture. This is why the Pmin values for air and oxygen mixtures are significantly different. Similar considerations should be taken into account in the critical size of the reaction vessel.
CONCLUSIONS
In this paper, we presented the results of experimental studies of the concentration limits of flame propagation in gas mixtures, in which combustible gases are hydrogen, methane, and propane, and the oxidant is air or oxygen, at reduced pressures in reaction vessels of various sizes. The minimal pressures Pmin of gas mixtures, below which flame propagation is impossible, were determined. The Pmin values are shown to depend on the type of fuel and oxidizer and on the ignition energy. At sufficiently large values of the ignition energy E, and the values of Pmin were found not to depend on E and to be determined by the composition of the combustible mixture. A qualitative interpretation of the obtained results is presented, based on ideas about the formation of a flame front during the ignition of combustible gas mixtures by an electric spark.
REFERENCES
V. I. Makeev, G. E. Golinevich, and A. N. Baratov, in Flammability and Chemical Extinguishing Agents (VNIIPO, Moscow, 1973), No. 4, p. 38 [in Russian].
M. Esseghir and C. E. Polymeropoulos, Combust. Flame 73, 99 (1988).
J. Elston and P. Laffitte, C.R. Acad. Sci. 225, 1313 (1947).
J. Legrand, R. Delbourgo, and P. Laffitte, C.R. Acad. Sci. 249, 1514 (1959).
J. D. Thomson and J. D. Enloe, Combust. Flame 10, 393 (1966).
P. Laffitte and R. Delbourgo, in Proceedings of the 4th International Symposium on Combustion (The Combust. Inst., Pittsburgh, 1953), p. 114.
V. K. Manzhos and S. G. Alekseev, in Proceedings of the 11th International Symposium on Combustion, Poland, 1989, p. 86.
S. G. Alekseev, V. K. Manzhos, and A. L. Dmitriev, Khim. Fiz. 9, 1599 (1990).
T. Cote et al., Combust. Sci. Technol. 48, 151 (1986).
E. Branoles, et al., in Proceedings of the 9th International Symposium on Loss Prevention and Safety Promotion in the Process Industries, Barcelona, 1998, p. 590.
A. Ya. Korol’chenko, et al., Khim. Fiz. 11, 258 (1992).
K. N. Homann and R. Poss, Combust. Flame 18, 300 (1972).
A. L. Dmitriev and G. S. Potekhin, in Combustion Processes and Fire Extinguishing Problems, Proceedings of the 3rd All-Union Conference (VNIIPO, Moscow, 1973), p. 143.
B. V. Karpinskii, G. I. Ksandopulo, and G. S. Potekhin, in Combustion Processes and Fire Extinguishing Problems, Proceedings of the 4th All-Union Conference (VNIIPO, Moscow, 1975), p. 15.
B. Lewis and G. von Elbe, Combustion, Flames and Explosions of Gases (Academic, New York, 1961).
S. A. Yantovskii and M. V. Chernyak, Zh. Fiz. Khim. 40, 2899 (1966).
Ya. B. Zel’dovich, Selected Works. Chemical Physics and Hydrodynamics (Nauka, Moscow, 1984) [in Russian].
V. I. Potyakin, A. S. Melikhov, and B. A. Ivanov, in Burning Heterogeneous and Gas Systems, Proceedings of the 8th All-Union Symposium on Combustion and Explosion (IKhF AN SSSR, Moscow, 1985), p. 85.
A. L. Furno, et al., in Proceedings of the 13th International Symposium on Combustion (The Combust. Inst., Pittsburgh, 1971), p. 593.
B. A. Ivanov, Physics of Acetylene Explosion (Khimiya, Moscow, 1969) [in Russian].
Ya. B. Zel’dovich and V. V. Voevodskii, Thermal Explosion and Flame Propagation in Gases (Kazakh. Univ., Alma-Ata, 2004) [in Russian].
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Ivanov
Rights and permissions
About this article
Cite this article
Glukhov, I.S., Shebeko, Y.N., Shebeko, A.Y. et al. Limiting Conditions of Flame Propagation in Gas Mixtures at Reduced Pressures. Russ. J. Phys. Chem. B 13, 471–477 (2019). https://doi.org/10.1134/S1990793119030047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793119030047