Abstract—
In this work we have studied the effect of the phytoecdysteroid 20-hydroxyecdysone (20E) on the functioning of mouse skeletal muscle mitochondria. It is shown that 20E at a concentration of 100 µM or more suppresses mitochondrial respiration fueled by glutamate and malate (substrates of complex I of the respiratory chain) or succinate (substrate of complex II of the respiratory chain). This effect of 20E is accompanied by a decrease in the mitochondrial membrane potential and is associated with inhibition of the activity of complex III, the total activity of complexes I + III and II + III of the mitochondrial respiratory chain. We have noted a prooxidant effect of 20E, which manifests itself in an increase in the production of hydrogen peroxide by skeletal muscle mitochondria. In addition, 20E reduces the ability of mitochondria to accumulate calcium ions in the matrix. We discuss the mechanisms of the possible toxic effect of 20E on the functioning of skeletal muscle mitochondria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Ecdysteroids are a large family of polyhydroxylated invertebrate steroid hormones that regulate molting, metamorphosis, and reproduction in arthropods [1]. These substances are also synthesized in 5–6% of plant species (phytoecdysteroids), possibly as a defense against phytophagous insects [2]. More than 570 different ecdysteroids have been identified in nature [3], but the most studied is 20-hydroxyecdysone (20E, Fig. 1). In vivo and in vitro studies have revealed the beneficial effects of 20E on mammals: anabolic, hypolipidemic, antidiabetic, anti-inflammatory, hepatoprotective, etc. [3, 4].
Although ecdysteroids do not bind to vertebrate steroid receptors and their mechanism of action is still unknown, they are credited with many beneficial pharmacological properties [5–7]. Increases in body, organ and muscle mass, and protein synthesis have been reported following oral or intraperitoneal administration of ecdysteroids in several animal species: Japanese quail [8], mice [9], rats [10], and pigs [11]. Recent studies [12] have shown that 20E also has antitumor activity in cultured non-small cell lung cancer cells. In addition, ecdysterone can inhibit the growth of breast cancer cells by suppressing glycolysis and mitochondrial bioenergetics and inducing autophagy and apoptosis of cancer cells, without affecting healthy ones [13].
A number of studies indicate that mammalian mitochondria may be a target of 20E. Ecdysterone has been shown to have therapeutic effects in pathologies associated with mitochondrial dysfunction and oxidative stress [14–16]. Moreover, the beneficial effects of 20E are often associated with modulation of mitochondrial function, but it is unclear whether its mitochondrial effects are direct or mediated by activation of other cellular processes. It can be assumed that 20E is capable of influencing cellular bioenergetics and the functioning of mitochondria, which could have an impact on the functioning of cells and the whole organism. In 2015, Parr et al. showed that 20E exerts its effects in mammalian cells through interaction with estrogen receptor beta (ER-β) [17]. It is well known that activation of estrogen receptors has a positive effect on mitochondrial function. In particular, activation of estrogen receptors by 17β-estradiol may protect human neuroblastoma cells from ATP depletion, decreased mitochondrial membrane potential, and production of reactive oxygen species [18]. Nilsen et al. showed that 17β-estradiol administration protected primary hippocampal neurons from glutamate excitotoxicity by stimulating Bcl-2 expression and promoting mitochondrial tolerance to calcium overload [19]. Burstein et al. showed that ER-β can also modulate the opening of the mitochondrial permeability transition pore (MPT pore), which has an important impact on the ability of these organelles to accumulate calcium ions [20].
Previously, our laboratory studied the effect of a wide range of natural polycyclic compounds and, in particular, triterpenoids of the lupane, fusidane, and oleanane series, similar to phytoecdysteroids, on the functioning of isolated mitochondria [21–23]. It has been established that, depending on the structure and hydrophobicity, these compounds suppress respiration and oxidative phosphorylation of mitochondria with varying effectiveness, inhibiting the activity of respiratory chain complexes, and show protonophore and prooxidant effects [21–23]. These mitochondria-targeted effects appear to underlie the cytotoxicity of these compounds.
One could assume that 20E, being hydrophobic, is also capable of interacting with mitochondrial membranes and changing the functional activity of organelles. Therefore, in this work, we studied the effect of 20E on the functioning of mitochondria isolated from skeletal muscles of C57BL/10 mice. We have found that 20E dose-dependently reduces the parameters of oxidative phosphorylation of mitochondria and the membrane potential of organelles, which is due to inhibition of the activity of the respiratory chain complexes of these organelles. This is accompanied by an increase in the production of hydrogen peroxide by mitochondria, as well as a suppression of the ability of organelles to absorb and retain calcium ions in the matrix.
MATERIALS AND METHODS
Isolation of mitochondria from mouse skeletal muscles. Mitochondria were isolated from skeletal muscles of C57BL/10 mice (animal’s weight was 25–28 g) by differential centrifugation [24]. The muscles (quadriceps muscles of both hind limbs) were quickly removed and immersed in 5 mL of ice-cold phosphate-buffered saline (2.7 mM KCl, 1.5 mM KH2PO4, 136.9 mM NaCl, 8.9 mM Na2HPO4, pH 7.4) supplemented with 10 mM EDTA. The muscles were then cut into small pieces and visible fatty tissue, connective tissue, and ligaments were removed. Dissected muscles were resuspended in 5 mL of ice-cold phosphate-buffered saline supplemented with 10 mM EDTA and 0.05% trypsin for 30 min, then centrifuged for 5 min at 200 g. The suspension was homogenized in a medium containing 67 mM sucrose, 50 mM KCl, 10 mM EDTA, 0.2% bovine serum albumin (BSA) and 50 mM Tris–HCl buffer (pH 7.4) using an Ultra-Turrax T 10 basic rotary homogenizer (IKA, Japan) and transferred to a Potter homogenizer (ratio of tissue mass to average volume 1 : 8). The homogenate was centrifuged for 10 min at 700 g. Mitochondria from the supernatant were pelleted by centrifugation for 10 min at 8000 g, resuspended in 5 mL of medium containing 250 mM sucrose and 10 mM Tris–HCl buffer (pH 7.4), and centrifuged again for 10 min at 8000 g. Mitochondrial protein concentration was determined by the Bradford method. During the experiment, the mitochondrial suspension (20–30 mg of mitochondrial protein per 1 mL) was stored on ice.
Evaluation of respiration and oxidative phosphorylation of mitochondria. Mitochondrial respiration was recorded by the polarographic method using a Clark type oxygen electrode and an Oxygraph+ setup (Hansatech Instruments, UK) at 25°C and continuous stirring [25]. The incubation medium contained 200 mM sucrose, 20 mM KCl, 0.5 mM EGTA, 5 mM KH2PO4, and 10 mM HEPES–KOH, pH 7.4. The following concentrations of substrates and other reagents were used: 2.5 mM potassium malate, 2.5 mM potassium glutamate, 5 mM succinic acid, 0.2 mM ADP, 50 μM 2,4-dinitrophenol, and 1 μM rotenone. Substrate oxidation rates were expressed in nmol O2/min/mg mitochondrial protein. Mitochondrial respiration was assessed in the basal metabolic state (i.e., in the presence of exogenous substrates or state 2; in state 3, ADP-stimulated respiration; state 4, the state of mitochondria after all added ADP has been consumed in the process of ATP synthesis; respiration rate mitochondria in the 3UDNP state, mitochondrial respiration in the presence of the uncoupler 2,4-dinitrophenol at a concentration of 50 μM, causing maximum stimulation of respiration. Respiratory control (RC = state 3/state 4) and ADP/O ratio were determined according to [26]. The concentration of mitochondrial protein in the cell was 0.5 mg/mL.
Assessment of the activity of the respiratory chain complexes of skeletal muscle mitochondria. The effect of 20E on the activity of the electron transport chain complexes of skeletal muscle mitochondria was assessed using specific redox reactions according to the protocol [27] using a Multiskan GO plate spectrophotometer (Thermo Fisher Scientific, USA). Measurements were performed on destroyed mitochondria subjected to three freeze/thaw cycles at ‒20/+30°C in a hypotonic buffer containing 10 mM Tris–HCl (pH 7.6). The effect of 20E on the activity of respiratory chain complexes was expressed as a percentage of the average activity recorded in a series of control experiments. The mitochondrial protein concentration was 20 μg/mL.
Measurement of mitochondrial membrane potential (∆ψ). The electrical potential difference (Δψ) on the inner membrane of mitochondria was assessed by the distribution of the safranin O fluorescent probe (λex = 520 nm; λem = 580 nm) through the inner membrane using a Varioskan LUX plate spectrofluorimeter (Thermo Fisher Scientific, USA) [28]. Mitochondria (0.5 mg/mL) were incubated in a medium containing 210 mM mannitol, 70 mM sucrose, 1 mM KH2PO4, 10 μM safranin O, 10 μM EGTA, and 10 mM HEPES–KOH, pH 7.4. The following concentrations of substrates and other reagents were used: 2.5 mM potassium malate, 2.5 mM potassium glutamate, 5 mM succinic acid, 0.2 mM ADP, 50 μM 2,4-dinitrophenol, and 1 μM rotenone. The concentration of mitochondrial protein in the cell was 0.5 mg/mL.
Determination of calcium retention capacity of skeletal muscle mitochondria. The transport of Ca2+ across the inner mitochondrial membrane was monitored spectrophotometrically with an arsenazo III (2,2′-(1,8-Dihydroxy-3,6-disulfonaphthylene-2,7-bisazo)bisbenzenearsonic acid, 2,7-Bis(2-arsonophenylazo)chromotropic acid) indicator at 675–685 nm using Multiskan GO plate reader (Thermo Fisher Scientific, USA) at 25°C under constant stirring [29]. The incubation medium contained 210 mM mannitol, 70 mM sucrose, 1 mM KH2PO4, 50 μM arsenazo III, 10 μM EGTA, 10 mM HEPES–KOH, pH 7.4. The following concentrations of substrates and other reagents were used: 2.5 mM potassium malate, 2.5 mM potassium glutamate, 5 mM succinic acid, 0.2 mM ADP, 50 μM 2,4-dinitrophenol, and 1 μM rotenone. The concentration of mitochondrial protein was about 0.25 mg/mL. To determine the ability of mitochondria to retain Ca2+, 20 μM CaCl2 was added into the reaction medium successively. After several additions, external [Ca2+] increased, indicating a massive release of the ion from the organelles due to the opening of the Ca2+-dependent MPT pore. The ability of Ca2+ to induce pore opening in the mitochondria quantified as the calcium retention capacity (CRC) of mitochondria, i.e., the maximum amount of Ca2+ that can be accumulated in the matrix without subsequent induction of permeability transition.
Estimation of hydrogen peroxide production by mouse skeletal muscle mitochondria. The rate of Н2O2 production was measured using a test system including a fluorescent indicator Amplex Red and horseradish peroxidase on a Varioskan LUX plate spectrofluorimeter (Thermo Fisher Scientific, USA) at excitation and emission wavelengths of 560 and 590 nm, respectively [23]. The incubation medium contained 210 mM mannitol, 70 mM sucrose, 1 mM KH2PO4, 10 μM EGTA, 10 mM HEPES–KOH (pH 7.4), 10 μM Amplex Red and horseradish peroxidase (1 a.u./mL). The concentration of mitochondrial protein in the cuvette was 0.1 mg/mL. The measurements were carried out at 37°C and constant stirring. The amount of the resulting hydrogen peroxide was calculated from the calibration curve. A standard H2O2 solution was prepared on the day of experiment; its concentration was determined using the molar absorption coefficient E240 = 43.6 M−1 cm−1.
Statistical analysis. The data were analyzed using GraphPad Prism 5 and Microsoft Excel software and were presented as means ± SEM. The data obtained were statistically processed using Mann–Whitney U-test. Differences between groups were considered statistically significant at p < 0.05.
RESULTS AND DISCUSSION
It was previously shown that long-term (1 h) incubation of isolated rat liver mitochondria with 20E leads to inhibition of respiration and oxidative phosphorylation of isolated rat liver mitochondria fueled by glutamate and malate [16]. Table 1 shows that under conditions applied in our experiments, 20E also dose-dependently reduces the respiration rate of mouse skeletal muscle mitochondria in state 3, but a significant effect is observed only at the 20E concentration of 100 μM. In this case, we noted a 1.2-fold decrease in the respiration rate in state 3 when organelles were energized with glutamate and malate (substrates of complex I of the respiratory chain) and a 1.1-fold decrease when mitochondria were energized with succinate (substrate of complex II of the respiratory chain) in the presence of rotenone. This is accompanied by a decrease in respiratory control ratio of mitochondria, reflecting the efficiency of coupling of respiration and phosphorylation in mitochondria, by 1.1 times when organelles are energized by substrates of complex I or complex II. One can also note a tendency to decrease the respiration rate in the presence of the protonophore uncoupler DNP and the ADP/O ratio, reflecting the efficiency of ATP synthesis.
A further increase in the concentration of 20E does not lead to significant changes in the determined parameters. There is a tendency for a subsequent decrease in the rate of mitochondrial respiration in state 3 and a decrease in respiratory control ratio, however, the addition of 200 μM of 20E apparently leads to a saturation phase and a further increase in concentration does not lead to an enhanced effect.
It is known that suppression of respiration and oxidative phosphorylation in mitochondria is accompanied by a decrease in themitochondrial membrane potential (Δψ). Figure 2 shows that at a concentration of 10 μM 20E has no effect on the Δψ either when using glutamate and malate or succinate as respiration substrates. However, an increase in its concentration to a total of 20 μM and further is accompanied by a dose-dependent release of the fluorescent probe safranin O from mitochondria, which indicates a decrease in Δψ. The results obtained differ from the data obtained by Baev et al. [16], who showed that long-term preincubation of isolated rat liver mitochondria with 20E increases the mitochondrial membrane potential by 6–12% depending on the concentration and experimental conditions. However, it is important to note that this conclusion was made on the basis of calculations that take into account the subsequent depolarization of mitochondria by the CCCP uncoupler. In this case, the addition of 20E to isolated mitochondria had no significant effect on the fluorescence of rhodamine 123 used to measure Δψ, indicating that 20E had no effect on the membrane potential of liver mitochondria.
Effect of 20E on the membrane potential of mouse skeletal muscle mitochondria fueled by glutamate/malate (a) or succinate (b). Substrates and reagents: 2.5 mM potassium malate, 2.5 mM potassium glutamate (a), 5 mM succinic acid, 1 μM rotenone (b). The data of typical experiments obtained on a single preparation of mitochondria are presented. Similar results were obtained in two other independent experiments.
One of the reasons for the decrease in the efficiency of oxidative phosphorylation and membrane potential of mitochondria under the influence of 20E may be the suppression of the activity of complexes of the mitochondrial respiratory chain and its mobile components (coenzyme Q and cytochrome c), which is typical for a wide range of steroid compounds. Therefore, in the next part of the work, we studied the effect of 20E on the activity of individual complexes of the respiratory chain of mouse skeletal muscle mitochondria, as well as the total activity of complexes I + III and II + III, which allows to evaluate the mobility of coenzyme Q between these individual complexes. Figure 3 shows that 20E has no effect on the activity of complexes I, II, and IV of the respiratory chain, but dose-dependently reduces the activity of complex III and complexes II + III of the mitochondrial respiratory chain (by 14 and 13%, respectively, at a maximum concentration of 200 μM). The greatest effectiveness of 20E was noted in relation to the total activity of complexes I + III. In this case, already 50 µM of this agent caused a decrease in activity by 24%. Half-maximal inhibition of the activity of complexes I + III was achieved at 200 μM 20E. The observed effect may indicate that 20E is able to reduce the mobility of coenzyme Q between complexes I and III of the respiratory chain, and also less effectively between complexes II and III. In addition, ecdysterone also has an inhibitory effect on the activity of complex III of the mitochondrial respiratory chain, thereby reducing the efficiency of electron transfer from coenzyme Q to cytochrome c. Ecdysteroids are lipophilic molecules and, according to some data [30], can be incorporated into the membrane bilayer and thus affect the function of membrane proteins. It is possible that addition of 20E directly to mitochondria allows molecules of this compound to act on their inner membrane and suppress the activity of localized complexes of the mitochondrial respiratory chain.
Activity of mitochondrial respiratory chain complexes in the absence and presence of different concentrations of 20-hydroxyecdysone (values in % of activity compared with control). The activity values in the absence of 20E were taken as 100%. The results are presented as means ± SEM (n = 4). *, p < 0.05 vs. control (absence of 20Е).
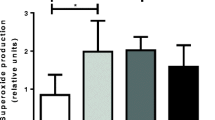
Mitochondria are one of the main producers of ROS in cells [31]. In this case, inhibition of respiratory chain complexes, realized through various mechanisms, has a significant impact on the intensity of ROS generation. In this work, we also assessed the effect of 20E on the rate of H2O2 production by mouse skeletal muscle mitochondria. One can see that in the case of energization of organelles with glutamate and malate, 20E at a maximum concentration of 100 μM causes an increase in the production of hydrogen peroxide (Fig. 4a), while under conditions of succinate-driven respiration this effect was not observed (Fig. 4b). It can be assumed that the pro-oxidant effect of 20E is associated both with a decrease in the mobility of coenzyme Q between complexes I and III, II and III, and with inhibition of the activity of complex III of the respiratory chain. These segments of the mitochondrial respiratory chain are known to be involved in the generation of ROS, and inhibition of their activity enhances ROS production. This is consistent with data indicating the generation of superoxide radicals in vitro in the presence of phytoecdysteroids [32]. On the other hand, it is important to note that many studies have shown that ecdysteroids, on the contrary, have antioxidant properties [33–36]. In particular, the antioxidant effect of 20E was studied in several in vitro systems [33]: a decrease in the intensity of lipid peroxidation in rat liver mitochondria under the influence of this agent was noted. Our results suggest that the antioxidant effect of 20E is not associated with its direct effect on mitochondrial targets.
20E is known as a modulator of Ca2+ homeostasis in insect cells [37]. Mammalian mitochondria play an important role in the regulation of intracellular calcium [38, 39] and changes in their functional activity have a significant impact on mitochondrial Ca2+ homeostasis. In this study, we assessed the effect of 20E on the ability of skeletal muscle mitochondria to absorb and retain calcium ions in the matrix. Figure 5 shows the results of a comparative study of the kinetics of Ca2+ uptake by mouse skeletal muscle mitochondria fueled by glutamate/malate (Fig. 5a) or succinate (Fig. 5b), incubated in the absence and presence of 20-hydroxyecdysone. It can be seen that mouse skeletal muscle mitochondria, energized by glutamate/malate, completely absorb Ca2+ when CaCl2 is added 4 times, 20 μM in each addition. (Fig. 5a). In this case, only the fifth addition of CaCl2 causes spontaneous release of Ca2+ from mitochondria (Fig. 5a), indicating the induction of a calcium dependent MPT pore in the inner mitochondrial membrane. Pre-incubation of mitochondria in the presence of 20E at concentrations of 20, 50, and 100 µM does not have a significant effect on the ability of mitochondria to absorb calcium ions (Fig. 5a). In the case of succinate-driven respiration, pre-incubation of organelles with 100 µM 20E leads to a decrease in the ability of mitochondria to absorb calcium ions, while 20 µM and 50 µM 20E do not have a similar effect (Fig. 5b). The ability of Ca2+ to induce the opening of MPT-pore in mitochondria can be expressed quantitatively as the calcium retention capacity of mitochondria or the maximum amount of Ca2+ that can be accumulated in the matrix without subsequent opening of the pore [40]. As shown in Fig. 5c, 20E does not affect this parameter of mitochondria when organelles are energized with glutamate and malate at all tested concentrations. At the same time, in the case of succinate-driven respiration, 100 μM 20E causes a significant decrease in the calcium retention capacity of mouse skeletal muscles mitochondria compared to the control (Fig. 5d). The results obtained indicate that the suppression of the functional activity of mitochondria caused by 20E also contributes to a decrease in the ability of mitochondria to effectively accumulate calcium ions in the matrix.
Effect of 20E on calcium transport in mouse skeletal muscle mitochondria. Uptake of Ca2+ supplements (20 µM pulses) by skeletal muscle mitochondria fueled by glutamate/malate (a) and succinate (b) in the absence (control) and presence of 20E. Calcium retention capacity of mouse skeletal muscle mitochondria fueled by glutamate/malate (c) or succinate (d) in the absence (control) and in the presence of various concentrations of 20E. *, p < 0.05 vs. control.
Thus, the results of this study suggest that the potential therapeutic effects of phytoecdysteroids and 20E in particular are more likely to occur through specific steroid receptors, while cytotoxic effects may occur at higher concentrations of 20E, including through a direct effect on the functional activity of mitochondria. 20E is able to suppress the functioning of mitochondrial respiratory chain complexes and reduce the efficiency of oxidative phosphorylation. In addition, this is accompanied by a decrease in the membrane potential of mitochondria, an increase in the production of ROS, as well as inhibition of the ability of organelles to accumulate calcium ions. Under in vivo conditions, these effects of 20E on skeletal muscle mitochondria may affect the ability of the organelle to synthesize ATP, which is necessary primarily for the contraction of skeletal muscles, as well as calcium homeostasis, which plays an important role in the correct regulation of the cycles of contraction and relaxation of muscle fibers. The data obtained should be taken into account when interpreting the results of in vivo experiments.
ABBREVIATIONS AND NOTATION
20E 20-hydroxyecdysone
ER-β estrogen receptor beta
BSA bovine serum albumin
MPT pore mitochondrial permeability transition pore
Δψ mitochondrial membrane potential
CRC calcium retention capacity
ROS reactive oxygen species
RCR respiratory control ratio
REFERENCES
Koolman J. 1989. Ecdysone: From chemistry of mode of action. Stuttgart: Thieme Verlag.
Toth N., Szabo A., Kacsala P., Heger J., Zador E. 2008. 20-Hydroxyecdysone increases fiber size in a muscle-specific fashion in rat. Phytomedicine. 15 (9), 691–698.
Lafont R., Harmatha J., Marion-Poll F., Dinan L., Wilson I.D. 2002. The ecdysone handbook. 3rd ed. Prague: Cybersales.
Savchenko R.G., Veskina N.A., Odinokov V.N., Benkovskaya G.V., Parfenova L.V. 2022. Ecdysteroids: Isolation, chemical transformations, and biological activity. Phytochem. Rev. 21, 1445–1486. https://doi.org/10.1007/s11101-021-09792-y
Bathori M., Toth N., Hunyadi A., Marki A., Zador E. 2008. Phytoecdysteroids and anabolic–androgenic steroids – structure and effects on humans. Curr. Med. Chem. 15, 75–91.
Slama K., Lafont R. 1995. Insect hormones—ecdysteroids: Their presence and actions in vertebrates. Eur. J. Entomol. 92, 355–377.
Dinan L., Lafont R. 2006. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J. Endocrinol. 191 (1), 1–8. https://doi.org/10.1677/joe.1.06900
Slama K., Koudela K., Tenora J., Mathova A. 1996. Insect hormones in vertebrates: Anabolic effects of 20‑hydroxyecdysone in Japanese quails. Experientia. 52, 702–706.
Stopka P., Stancl J., Slama K. 1999. Effect of insect hormone, 20-hydroxyecdysone on growth and reproduction in mice. Acta Soc. Zool. Bohemicae. 63, 367–378.
Syrov V.N. 2000. Comparative experimental investigations of the anabolic activity of phytoecdysteroids and steranabols. Pharm. Chem. J. 34, 193–197.
Kratky F., Opletal L., Hejhalek J., Kucharova S. 1997. Effect of 20-hydroxyecdysone on the protein synthesis of pigs. Zivocisna Vyroba. 42, 445–451.
Shuvalov O., Kirdeeva Y., Fefilova E., Netsvetay S., Zorin M., Vlasova Y., Fedorova O., Daks A., Parfenyev S., Barlev N. 2023. 20-Hydroxyecdysone confers antioxidant and antineoplastic properties in human non-small cell lung cancer cells. Metabolites. 13, 656. https://doi.org/10.3390/metabo13050656
Romaniuk-Drapała A., Lisiak N., TotonE., Matysiak A., Nawrot J., Nowak G., Kaczmarek M., Rybczyńska M., Rubiś B. 2021. Proapoptotic and proautophagic activity of 20-hydroxyecdysone in breast cancer cells in vitro. Chem. Biol. Interact. 342, 109479. https://doi.org/10.1016/j.cbi.2021.109479
Xia X., Zhang Q., Liu R., Wang Z., Tang N., Liu F., Huang G., Jiang X., Gui G., Wang L., Sun X. 2014. Effects of 20-hydroxyecdysone on improving memory deficits in streptozotocin-induced type 1 diabetes mellitus in rat. Eur. J. Pharmacol. 5, 740, 45–52. https://doi.org/10.1016/j.ejphar.2014.06.026
Mallek A., Movassat J., Ameddah S., Liu J., Semiane N., Khalkhal A., Dahmani Y. 2018. Experimental diabetes induced by streptozotocin in the desert gerbil, Gerbillus gerbillus, and the effects of short-term 20-hydroxyecdysone administration. Biomed. Pharmacother. 102, 354–361. https://doi.org/10.1016/j.biopha.2018.03.070
Baev A.Y., Charishnikova O.S., Khasanov F.A., Nebesnaya K.S., Makhmudov A.R., Rakhmedova M.T., Khushbaktova Z.A., Syrov V.N., Levitskaya Y.V. 2022. Ecdysterone prevents negative effect of acute immobilization stress on energy metabolism of rat liver mitochondria. J. Steroid Biochem. Mol. Biol. 219, 106066. https://doi.org/10.1016/j.jsbmb.2022.106066
Parr M.K., Botre F., Nass A., Hengevoss J., Diel P., Wolber G. 2015. Ecdysteroids: A novel class of anabolic agents? Biol. Sport. 32, 169–173.
Wang J., Green P.S., Simpkins J.W. 2001. Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. J. Neurochem. 77 (3), 804–811. https://doi.org/10.1046/j.1471-4159.2001.00271.x
Nilsen J., Diaz Brinton R. 2003. Mechanism of estrogen-mediated neuroprotection: Regulation of mitochondrial calcium and Bcl-2 expression. Proc. Natl. Acad. Sci. USA. 100 (5), 2842–2847. https://doi.org/10.1073/pnas.0438041100
Burstein S.R., Kim H.J., Fels J.A., Qian L., Zhang S., Zhou P., Starkov A.A., Iadecola C., Manfredi G. 2018. Estrogen receptor beta modulates permeability transition in brain mitochondria. Biochim. Biophys. Acta. Bioenerg. 1859 (6), 423–433. https://doi.org/10.1016/j.bbabio.2018.03.006
Dubinin M.V., Ilzorkina A.I., Salimova E.V., Landage M.S., Khoroshavina E.I., Gudkov S.V., Belosludtsev K.N., Parfenova L.V. 2023. Effect of fusidic acid and some nitrogen-containing derivatives on liposomal and mitochondrial membranes. Membranes (Basel). 13 (10), 835. https://doi.org/10.3390/membranes13100835
Dubinin M.V., Nedopekina D.A., Ilzorkina A.I., Semenova A.A., Sharapov V.A., Davletshin E.V., Mikina N.V., Belsky Y.P., Spivak A.Y., Akatov V.S., Belosludtseva N.V., Liu J., Belosludtsev K.N. 2023. Conjugation of triterpenic acids of ursane and oleanane types with mitochondria-targeting cation F16 synergistically enhanced their cytotoxicity against tumor cells. Membranes (Basel). 13 (6), 563. https://doi.org/10.3390/membranes13060563
Dubinin M.V., Semenova A.A., Ilzorkina A.I., Mikheeva I.B., Yashin V.A., Penkov N.V., Vydrina V.A., Ishmuratov G.Y., Sharapov V.A., Khoroshavina E.I., Gudkov S.V., Belosludtsev K.N. 2020. Effect of betulin and betulonic acid on isolated rat liver mitochondria and liposomes. Biochim. Biophys. Acta. Biomembr. 1862 (10), 183383. https://doi.org/10.1016/j.bbamem.2020.183383
Dubinin M.V., Talanov E.Y., Tenkov K.S., Starinets V.S., Mikheeva I.B., Sharapov M.G., Belosludtsev K.N. 2020. Duchenne muscular dystrophy is associated with the inhibition of calcium uniport in mitochondria and an increased sensitivity of the organelles to the calcium-induced permeability transition. Biochim. Biophys. Act-a. Mol. Basis Dis. 1866 (5), 165674. https://doi.org/10.1016/j.bbadis.2020.165674
Dubinin M.V., Svinin A.O., Vedernikov A.A., Sta-rinets V.S., Tenkov K.S., Belosludtsev K.N., Samartsev V.N. 2019. Effect of hypothermia on the functional activity of liver mitochondria of grass snake (Natrix natrix): Inhibition of succinate-fueled respiration and K+ transport, ROS-induced activation of mitochondrial permeability transition. J. Bioenerg. Biomembr. 51 (3), 219–229.
Chance B., Williams G.R. 1955. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J. Biol. Chem. 217 (1), 383–393.
Spinazzi M., Casarin A., Pertegato V., Salviati L., Angelini C. 2012. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 7 (6), 1235–1246. https://doi.org/10.1038/nprot.2012.058
Dubinin M.V., Sharapov V.A., Ilzorkina A.I., Efimov S.V., Klochkov V.V., Gudkov S.V., Belosludtsev K.N. 2022. Comparison of structural properties of cyclosporin A and its analogue alisporivir and their effects on mitochondrial bioenergetics and membrane behavior. Biochim. Biophys. Acta Biomembr. 864 (9), 183972.
Dubinin M.V., Talanov E.Y., Tenkov K.S., Starinets V.S., Mikheeva I.B., Belosludtsev K.N. 2020. Transport of Ca2+ and Ca2+-dependent permeability transition in heart mitochondria in the early stages of Duchenne muscular dystrophy. Biochim. Biophys. Acta. Bioenergetics. 1861 (10), 148250.
Lafont R., Dinan L. 2003. Practical uses for ecdysteroids in mammals including humans: An update. J. Insect. Sci. 3, 7. https://doi.org/10.1093/jis/3.1.7
Andreyev A.Y., Kushnareva Y.E., Murphy A.N., Starkov A.A. 2015. Mitochondrial ROS metabolism: 10 years later. Biochemistry (Mosc.). 80 (5), 517–531.
Shchulkin A.V., Yakusheva E.N., Davydov V.V., Darmogray V.N. 2012. The study of the direct antioxidant activity of phytoecdysterone in vitro. Rossiiski med.-biol. Vestnik imeni akad. I. P. Pavlova (Rus.). 1, 51–57.
Kuzmenko A.I., Niki E., Noguchi H. 2001. New functions of 20-hydroxyecdysone in lipid peroxidation. J. Oleo Sci. 50 (6), 497–506.
Cai Y.J., Dai J.Q., Fang J.G., Ma L.P., Hou L.F., Yang L., Liu Z.L. 2002. Antioxidative and free radical scavenging effects of ecdysteroids from Serratula strangulata. Can. J. Physiol. Pharmacol. 80 (12), 1187–1194. https://doi.org/10.1139/y02-152
Sahach V.F., Korkach Iu.P., Kotsiuruba A.V., Rudyk O.V., Vavilova H.L. 2008. Mitochondrial permeability transition pore opening inhibition by ecdysterone in heart mitochondria of aging rats. J. Phys. 54 (4), 3–10.
Das N., Mishra S.K., Bishayee A., Ali E.S., Bishayee A. 2021. The phytochemical, biological, and medicinal attributes of phytoecdysteroids: An updated review. Acta. Pharm. Sin. B. 11 (7), 1740–1766. https://doi.org/10.1016/j.apsb.2020.10.012
Li Y.B., Li X.R., Yang T., Wang J.X., Zhao X.F. 2016. The steroid hormone 20-hydroxyecdysone promotes switching from autophagy to apoptosis by increasing intracellular calcium levels. Insect. Biochem. Mol. Biol. 79, 73–86. https://doi.org/10.1016/j.ibmb.2016.10.004
Dubinin M.V., Belosludtsev K.N. 2019. Taxonomic features of specific Ca2+ transport mechanisms in mitochondria. Biochem. (Moscow) Suppl. Series A. Membr. Cell Biol. 13, 194–204.
Belosludtsev K.N., Dubinin M.V., Belosludtseva N.V., Mironova G.D. 2019. Mitochondrial Ca2+ transport: Mechanisms, molecular structures, and role in cells. Biochemistry. 84 (6), 593–607.
Rasola A., Bernardi P. 2011. Mitochondrial permeability transition in Ca2+-dependent apoptosis and necrosis. Cell Calcium. 50, 222–233.
Funding
The work was supported by Russian Science Foundation (project no. 23-75-01061).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflict of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All procedures were performed in accordance with the European Communities Council Directive (November 24, 1986; 86/609/EEC) and the Declaration on humane treatment of animals. The Protocol of experiments was approved by the Commission on Bioethics of the Mari State University.
Additional information
Translated by M. Dubinin
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Semenova, A.A., Igoshkina, A.D., Mikina, N.V. et al. The Effect of 20-Hydroxyecdysone on the Functioning of Isolated Mouse Skeletal Muscle Mitochondria. Biochem. Moscow Suppl. Ser. A 18, 127–135 (2024). https://doi.org/10.1134/S1990747824700144
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747824700144