Abstract—
Internal ultrastructure of the muscle tissue mitochondria of horsehair worm Gordionus alpestris (Nematomorpha) was studied using morphometry. Surface area of the inner mitochondrial membrane per unit of the mitochondrial volume, or surface density of the inner mitochondrial membrane, was measured as a main morphometric parameter. The surface density of the inner mitochondrial membrane of the G. alpestris muscle tissue was compared to the respective parameter of the skeletal and cardiac muscle mitochondria. The surface density of the inner mitochondrial membrane of the worm was close to the surface density values of the cardimyocytes of 3-month-old mice and Wistar rats and was slightly higher than the surface density of mitochondria from the skeletal muscle of 3-month-old mice. The functional significance of the well-developed system of mitochondrial membranes of extended mitochondria of the horsehair worm is discussed as a structure necessary to ensure effective functioning of the circomyarian conntractile apparatus in the muscle tissue of the horsehair worm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Our recent study of the ultrastructure of the muscle tissue chondriome in horsehair worm Gordionus alpestris revealed that mitochondrial apparatus consists of ribbon-like mitochondria up to 800 μm in length. They form well-developed system of elongated mitochondria located in the central cytoplasmic space and completely fill it [1]. However, according to the published data, chondriome of the horsehair worm muscle consists of a few small single mitochondria [2, 3]. We hypothesized that the system of elongated giant mitochondria in the horsehair muscle tissue is not just a specific morphological feature of this tissue, but it represents a functionally related type of chondriome organization, which determines the energy supply of actively working muscle tissue.
It is known that functional features of mitochondria are determined not only by morphological states but also by the parameters of the external and internal mitochondrial membrane. Estimation of such parameters is possible using methods of morphometry and stereology that allow establishing the correlation of mitochondrial structural parameters with the energy state of a single mitochondria and the whole tissue [4–9]. The energy state of the mitochondria is well described by such well-known morphometric parameter as a surface area of the inner mitochondrial membrane per unit of the mitochondrial volume, or surface density of the inner mitochondrial membrane [10, 11].
We took this parameter as a main morphometric criteria for the analysis of the inner mitochondrial ultrastructure of muscle tissue mitochondria in a horsehair worm. To validate our assumption about the association between special organization of the mitochondrial apparatus of the worm with the energy consuption rate and fuctional features, we compared surface density of the inner mitochondrial membrane in G. alpestris with the same parameter for mitochondria in skeletal and cardiac muscle of mice and rats.
MATERIALS AND METHODS
Animals. Gordionus alpestris (Villot, 1885) worms were collected in the Republic of Adygea, Nikel township (44°10′40.1″ N, 40°09′28.2″ E). The host is millipede Pachyiulus krivolutskyi. Horsehair worms parasitize in the bodies of various arthropods (mainly insects). After reaching a certain size, the worms leave their hosts and convert into free-living water organisms, which are, however, unable to feed and die shortly after copulation [12]. One female and one male specimens of the parasitic form and 2 females and 2 males of the free-living form were studied. Muscle tissue was isolated from the middle part of the worm body.Laboratory mice (male F1, C57Bl/6 ×CBA) were also used in our study. Animals were kept in standard vivarium conditions, in individually ventilated cells (IVC system, TECNIPLAST S. p. A., Italy), 5 amimals in each, with access to food (Ssniff Spezialdiaten GmbH, Germany) and water ad libitum, in pathogen-free conditions with 12/12 light cycle, at least 15 air changes per hour, temperature of 20–24°С, and humidity of 30–70%. Lignocell wood fibers were used as flooring (JRS, Germany). All materials contacting with the animals were sterillized. Tissues from thigh muscles (m. vastus lateralis, m. vastus medius, m. vastus intermedius) were taken for the skeletal muscle mitochondria investigation; tissue from the left heart ventricule was taken for investigation of cardiac mitochondrial ultrastructure. All materials were fixed with 3% glutaraldehyde solution (Sigma–Aldrich, USA) in 0.1 M phosphate buffer (pH 7.4) for 2 h at 4°C. Specimens were further fixed with 1% osmium tetroxide for 1.5 h and then dehydrated in alcohol series with increasing alcohol concentrations of 50, 60, 70, 80 and 96% (70% alcohol contained 1.4% uranyl acetate; Serva, Germany) to enhance contrast. After that, samples were embedded in Epon812 epoxy resin.A series of ultrathin sections was prepared with an ultramicrotome (Leica, Austria) and stained with lead. Obtained preparations were imaged and photographed by JEM1400 electron microscope (JEOL, Japan) operating at the accelerating voltage of 100 kV and beam current of 65 μA, equipped with a QUEMESA camera (Olympus, USA) and processed with the iTEM software provided with the electron microscope (EMSIS GmbH, Germany). For morphometric analysis, on each micrograph single mitochondria were outlined manually and followed by delineation of their inner membranes. Width of line used to delineate inner membranes was determined according to the known micrograph scale and assuming inner mitochondrial membrane width to be 75 Å and section thickness, ~700 Å. Various morphometric parameters, such as area of the inner mitochondrial membrane inside section volume, total length of the inner mitochondrial membrane, and volume of mitochondrial section, were calculated using Adobe Photoshop software (Adobe Systems, Inc., USA). On the basis of these data, the area of the inner mitochondrial membrane (µm2) per unit of volume of mitochondria (µm3) was calculated. Statistical processing of the morphometric data was performed using STATISTICA 8 software suite (StatSoft Inc., USA). Distribution normality was checked by the Kolmo-gorov–Smirnov test. Pairwise comparisons were performed between the two forms of horsehair warm, as well as between horsehair worm muscle and mice skeletal and cardiac muscle Significance level was checked using the Mann–Whitney test with Bonferroni correction for multiple testing.
RESULTS AND DISCUSSION
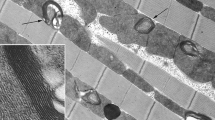
Muscle tissue of the horsehair worm (flattened circomyarian type) is represented by a layer of flattened ribbon-like longitudinal muscle fibers. The narrow cytoplasmic region in the center of muscle fiber is surrounded by a dense peripheral layer of contractile material forming an envelope around it. Figure 1a shows a fragment of muscle fiber cross section. Narrow central cytoplasmic space can be seen, uniformly filled by small mitochondria of round or elongated shape. In our previous study we found them to be cross sections of giant extended mitochondria reaching a length of more than 4 μm, aggregated into longitudinal strands. In Fig. 1b, at higher magnification, it is seen that mitochondria have clearly pronounced matrix and randomly arranged cristae. No difference in mitochondrial ultrastructure were observed between male and female specimens. Such ultrastructure suited well for morphometric analysis of surface density of the inner membrane of horsehair worm muscle mitochondria: cristae can be clearly seen in 8–10 mitochondrial cross-sections available for measurements.
Previously, we found no difference in mitochondrial ultrastructure of parasitic and free-living forms of horsehair worm. In this work, we took measurements in central cross sections of cytoplasmic region of muscle fibers in 20 electron microscopic images in both groups of animals.
Fig. 2 shows the result of surface density calculations for the inner mitochondrial membrane of muscle tissue mitochondria in horsehair worm. Mean values reached 39.42 μm2/μm3 for the parasitic form and 41.7 μm2/μm3 for the free-living form respectively. Standard error did not exceed 1.5 μm2/μm3; statistically significant differences between mean values were not detected, suggesting that muscle tissue mitochondria in both forms of the horsehair worm have similar activity levels.
The absence of differences in the mitochondrial structure in two horsehair lifeforms (both in this study and our previous work) can be due to the fact that a precise identification of the life-cycle stage of the studied animals is quite difficult.
It is interesting to compare the obtained morphometric data on muscle tissue mitochondria of horsehair worm with the results of the morphometric analysis for skeletal and cardiac mitochondria of mammals, capable of both synchronous and energy-demanding contraction of muscle cells. It is well-known that along with significant differences in operation of this muscle tissues (in contrast with the skeletal muscle, cardiac muscle performs contraction-relaxation cycle continuously) and serious differences in structural organization of cardiac and skeletal muscle chondriome, in both tissues it serves the same function of providing operation of contractile apparatus. It is achieved by membrane systems of giant mitochondria joined into unified mitochondrial reticulum.
Most of mitochondria in the skeletal muscle are known to be organized as mitochondrial reticulum, which is formed by giant branched mitochondria and located as layers in the isotropic zones, perpendicular to the long axis of muscle fiber [13, 14]. Therefore, on the longitudinal section of the muscle fiber, cross sections of giant mitochondria, which appear as small single mitochondria located pairwise in the isotropic zone on both sides of Z-line, can be seen as (Fig. 3)
Morphometric estimates of the surface density of the inner mitochondrial membrane in mouse skeletal muscle were based on 20 electron micrographs of the fragments of muscle longitudinal sections with at least 15 cross-sections of mitochondrial reticulum on it. As is seen in Fig. 2, surface density of the inner mitochondrial membrane in the skeletal muscle mitochondria reached 33.43 μm2/μm3, which is statistically lower than the values both for parasitic and free-living forms of the horsehair worm.
In cardiomyocytes, mitochondrial material is arranged in layers formed by mitochondria and joined into unified system of mitochondrial reticulum, surrounding bundles of myofibrills (Fig 4) [15, 16]. Surface density analysis of the inner membrane of cardiac mitochondria was also performed on 20 electron micrographs of longitudinal section fragments of mouse cardiomyocytes. Each fragment contained at least 10 cross sections of mitochondria.
Mean surface density of the inner membrane of mouse cardiac mitochondria reached 37.53 μm2/μm3 (Fig. 3). Similar values were obtained earlier for cardiac mitochondria of 3-months-old Wistar rats: 41.30 μm2/μm3 [8]. Thus, not only morphological ultrustructure studies, but also analysis of inner membrane morphometric parameters showed the presence of advanced mitochondrial membrane system in muscle tissue of Gordionus alpestris.
Obliquely striated muscle of the horsehair worm (flattened circomyarian type), demonstrates features similar to both smooth muscle cells (mitochondria are located in specific place, central cytoplasmic space, and not among myofibrills) and skeletal muscle, according to ultrastructure and features of functioning.
The structure of the mitochondrial apparatus of giant ribbon-like muscle fibers of the horsehair worm (according to published data) [2, 3] can be imagined as small single not connected mitochondria located in the central cytoplasmic space; then it will be obvious that under maximum contractile workload small single mitochondria with few cristae will fail to coordinate the energy production sufficient to fulfill the demands of extended muscle fibers reaching up to 800 μm in length.
Morphometric analysis has shown that the surface density of the inner mitochondrial membrane in muscle tissue of the horsehair worm corresponds to the values obtained for cardiac mitochondria of mice (C57Bl/6 × CBA, 3 month-old), cardiac mitochondria of 3 months-old Wistar rats, and is higher (statistically significant) than in skeletal muscle mitochondria of mice (C57Bl/6 × CBA, 3 month-old).
According to the published literature [2, 3], most muscles of the horsehair worm are of obliquely striated type. The so-called “oblique” sarcomeres are present in oblique-striated muscle cells, with Z disks staggered relative to the long axis of the cell. Unlike skeletal muscle, in obliquely-striated muscle cells, Z disks make up a system of dense fibrillar fragments arranged in a row and separated by free spaces, without rigid structural interconnection of thick myosin protofibrils in the center of the sarcomere. During the contraction, not only actin protofibrils move towards the center of the sarcomere, but also myosin protofibrils shift relative to each other, which leads to a change in the tilt angle of the sarcomere. As a result, the arngement of Z disks changes and they become perpendicular to the long axis of the cell, like in the sarcomeres of skeletal muscle. These structural features of obliquely-striated muscle allows generating more powerful tension (as compared to the skeletal muscle tissue), which is necessary for multidirectional contractions required for vermicular body movements of the horsehair worm [17]. Muscle contractions is well-known to be energy-dependent processes. Therefore, it can be assumed that statistically significant increase in the surface density of the inner mitochondrial membrane, which we observed in muscle tissue mitochondria of the horse hair worm, compared to mouse skeletal muscle, is necessary to sustain consistent level of the mitochondria respiration activity. This activity underlies features of mechanochemical contraction processes of the horsehair worm muscle cells.
Thus, the results of our study of the chondriome organization in muscle cells of the horsehair worm by ultrastuctural and morphometric analysis reveal the functional significance of the advanced mitochondrial membrane system of extended mitochondria as a structure necessary for the efficient operation of contractile apparatus of the horsehair worm Gordionus alpestris.
REFERENCES
Vays V.B., Vangeli I.M., Eldarov C.M., Bakeeva L.E., Efeykin B.D. 2019. Mitochondria in obliquely striated muscles of the horsehair worm Gordionus alpestris (Nematomorpha, Gordioidea) with structural organization typical of cells with energy-intensive processes. Biochemistry (Moscow). 84 (1), 56–61.
Lanzavecchia G., Valvassory R., Magda de Eguileor, Lanzavecchia P. 1979. Three-dimensional reconstruction of the contractile system of the Nematomorpha muscle fiber. J. Ultrastruct. Res. 66, 201–223.
Restelli M. A., de Villalobos L.C., Fernanda Z. 2002. Ultrastructural description of the musculature, the intraepidermal nervous system, and their interrelation in Pseudochordodes bedriagae (Nevatomorpha). Cell Tissue Res. 308, 299–306.
Weibel E.R. 1979. Stereological methods. Practical methods for biological morphometry. London, Academic Press.
Gundersen H.J., Bendtsen T.F., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J.R., Pakkenberg B., Sørensen F.B., Vesterby A. 1989. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 96 (5), 379–394.
Mandarim-de-Lacerda C.A. 2003. Stereological tools in biomedical research. An. Acad. Bras. Cienc. 75 (4), 469–486.
Shinderite V.S., Yasaitis A.A. 1984. Effects of energy metabolism inhibitors on the morphometric parameters and spatial structure of mitochondria in L cells. Tsitologya (Rus.). 26, 666–671.
Eldarov C.M., Vays V.B., Vangeli I.M., Kolosova N.G., Bakeeva L.E. 2015. Morphometric examination of mitochondrial ultrastructure in aging cardiomyocytes. Biochemistry (Moscow).80 (5), 604–609.
Nepomnyashchikh L.M., Lushnikova E.L., Molodykh N.A., Klinnikova M.G., Molodykh O.P. 2011. Ultrastructure and stereology of cardiomyocytes in the development of regenerative and plastic myocardial insufficiency during ontogeny. Bull. Exp. Biol. Med.151 (1), 88–94.
Mccallister B.D., Brown A.L. 1965. A quantitative morphological study of the mitochondria in experimental cardiac hypertrophy. Lab. Invest. 14, 692–700.
Cieciura L., Rydzynski K., Klitonczyk W. 1979. Stereologic studies on mitochondrial configuration in different organs of the rat. Cell Tissue Res. 196, 347–360.
Dogel V.A. 1975. Zoologia bespozvonochnykh (Zoology of invertebrates). M., Vysshaya Shkola.
Bakeeva L.E., Skulachev V.P., Chentsov Yu.S. 1977. Mitochonrial reticulum: Structure and possible functions of the new type intracellular organelles in muscle tissue. Vestnik Mosc. Univ., Ser. Biologia (Rus.). 3, 23–38.
Bakeeva L.E., Chentsov Yu.S., Skulachev V.P. 1978. Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim. Biophys. Acta.501, 349–369.
Bakeeva L.E., Chentsov Yu.S., Skulachev V.P. 1982. Intermitochondrial contacts in cardiomyocytes. Tsitologia (Rus.). 24 (2), 161–166.
Bakeeva L.E., Chentsov Yu.S., Skulachev V.P. 1983. Intermitochondrial contacts in myocardiocytes. J. Mol. Cell. Cardiol. 15, 413–420.
Zavarzin A.A. 1985. Osnovy sravnitel’noi gistologii (Fundamentals of comparative histology). L., Izd. Leningradskogo Universiteta, p. 321.
ACKNOWLEDGMENTS
The authors thank Dr. V.V. Aleshin for the assistance in the data interpretation and preparation of the manuscript. The work was supported by the Russian Foundation for Basic Research (project no. 18-34-00413).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
All procedures were performed in accordance with the protocol approved by the local bioethics comittee IC VEK OOO “Research Institute of Mitoengineering MSU” (resolution no. 67 of 28.04.2015), European Communities Council Directive (November 24, 1986; 86/609/EEC) and the Declaration on humane treatment of animals.
Additional information
Translated by Ch. Eldarov
Rights and permissions
About this article
Cite this article
Eldarov, C.M., Vays, V.B., Vangeli, I.M. et al. Morphometric Analysis of the Internal Ultrastructure of Mitochondria of Muscle Tissue in Horsehair Worm Gordionus alpestris (Nematomorpha). Biochem. Moscow Suppl. Ser. A 14, 255–259 (2020). https://doi.org/10.1134/S199074782002004X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199074782002004X