Abstract—
The effect of α,ω-hexadecanedioic acid (HDA) as an inducer of Ca2+-dependent permeability of the inner membrane (pore opening) of isolated rat liver mitochondria was studied in the absence and presence of pore blocker cyclosporin A (CsA) and one of its effective modulator – inorganic phosphate (Pi). It was shown that the addition of HDA at a concentration of 30 µM to Ca2+-loaded mitochondria induces swelling of the organelles, rapid Ca2+ release from the matrix, and almost total drop in Δψ, which indicates the induction of the Ca2+-dependent permeability of the inner membrane. It was found that 1 µM CsA or 1 mM Pi added separately do not affect these effects of HDA. At the same time, in the presence of both CsA and Pi, HDA added to Ca2+-loaded mitochondria does not induce their swelling, Ca2+ release from the matrix, and a drop in Δψ. It is found that unlike HDA, the induction of Ca2+-dependent lipid pore by palmitic acid is not blocked by the combined action of CsA and Pi. On the basis of the obtained data Pi is considered as a blocker of the HDA-induced Ca2+-dependent pore in the presence of CsA. In this case, Pi can not be replaced by a similar permeable anion vanadate. It was established that this effect of Pi was eliminated if mitochondria were incubated with SH-reagents mersalyl (10 nmol/mg protein) and n-ethylmaleimide (200 nmol/mg protein), which are known as Pi-carrier inhibitors. We conclude that the mechanisms of the effects of HDA and palmitic acid as inducers of the Ca2+-dependent permeability of liver mitochondria differ significantly. The Ca2+-dependent effect of HDA can be considered as the formation of a pore sensitive to the combined action of CsA and Pi, while the Ca2+-dependent effect of palmitic acid is the formation of a lipid pore. Possible causes of the blocking action of Pi on the HDA-induced Ca2+-dependent mitochondrial pore are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
α, ω-Dicarboxylic (syn. α, ω-dioic) acids are formed in the liver cells by ω-oxidation of the corresponding monocarboxylic fatty acids [1, 2]. Under normal physiological conditions, ω-oxidation of fatty acids in liver cells does not exceed 10% of their total metabolism and is considered as a minor metabolic pathway [1, 2]. However, under conditions of insufficient activity of the main pathways of fatty acid metabolism – β-oxidation in mitochondria and peroxisomes – a significant increase in their ω-oxidation is observed [1, 2]. In the course of ω-oxidation of one of the most common natural fatty acids – palmitic, α,ω-hexadecanedicarboxylic acid (HDA) is formed, its accumulation in blood and cells is observed under various pathological conditions associated with impaired lipid metabolism [2–5]. It is assumed that an increase in the level of α,ω-dicarboxylic acids in the body can be a factor favoring the death of cells of vital organs [4, 5].
One of the mitochondria-related steps in the mechanism of cell death is the induction of calcium-dependent nonspecific permeability of the inner membrane, resulting in the redistribution of ions and water-soluble substances with a molecular weight up to 1500 Da in accordance with their concentration gradient through the inner membrane of organelles (mitochondrial pore opening) [6, 7]. In turn, this leads to dissipation of energy stored in the form of the difference in electrochemical potentials of hydrogen ions on the inner membrane, impaired ATP synthesis, as well as the release of the so-called apoptogenic proteins, such as cytochrome c, apoptosis inducing factor, endonuclease G, from the intermembrane space into the cytoplasm [6, 7].
Various hypotheses about the mechanism of mitochondrial pore formation are known. According to an early hypothesis, the main component of the pore that forms the channel in the inner mitochondrial membrane is the ADP/ATP antiporter (syn. adenine nucleotide translocator) [8, 9]. Later, other proteins of the inner mitochondrial membrane were identified, claiming to be the main component of the pore: phosphate carrier [9] and F0F1-ATP synthase [10, 11]. The role of the last one in the pore formation, however, is the subject of discussion [12–14]. An important role in the pore formation is performed by one of the proteins of the matrix – cyclophilin D. Cyclosporin A (CsA), a specific blocker of such a “classical” pore, reduces the sensitivity of mitochondria to Ca2+ as a pore inducer, by binding to cyclophilin D [6–8]. At present, the hypothesis that the main structural unit of the mitochondrial pore is the F0F1-ATP synthase is widespread, while the other proteins of the inner membrane listed above act as protein modulators of the pore [7, 10, 11].
Among the low molecular weight modulators of the pore, inorganic phosphate (Pi) occupies a special place. It is well known that Pi, like its chemical analogues: inorganic arsenate and inorganic vanadate, increases the sensitivity of mitochondria to Ca2+ as a pore inducer [7, 15–17]. It is assumed that such an action of Pi and its analogs is due to the binding of magnesium ions in the matrix [7, 15]. It was also established that Pi enhances the effect of CsA as a pore blocker [7, 15–27]. In experiments on the liver mitochondria of mutant mice lacking cyclophilin D, the effect of Pi as a pore blocker in the absence of CsA was shown [15, 16]. It is assumed that there is a regulatory site on the inner surface of the inner membrane of mitochondria, the binding of Pi to this site is accompanied by inhibition of the pore, while in the absence of CsA it is masked by cyclophilin D [15].
Free monocarboxylic fatty acids are effective natural inducers of the Ca2+-dependent pore opening [15–23]. In early studies, it was found that the effect of fatty acids is manifested only in the presence of Ca2+ and is suppressed by CsA [15–18]. It is assumed that fatty acids can enhance the effect of Pi as a pore inducer by increasing the negative surface potential on the mitochondrial membrane [15]. Later it was found that the induction of the pore by palmitic and other saturated fatty acids is not suppressed by CsA, and for the induction of such a CsA-insensitive pore, Ca2+ can be replaced by ions of other bivalent metals, for example Sr2+ [22–24]. It was also shown that saturated monocarboxylic acids in the presence of Ca2+ can effectively induce nonspecific permeability of both artificial membranes and the erythrocyte plasma membrane, causing lysis of cells [22, 23]. It is assumed that the mechanism of induction of such permeability is based on the formation of lipid pores as a result of the formation of complexes of fatty acids and Ca2+ [22–25]. However, it has not been studied to what extent the effect of saturated fatty acid as an inducer of Ca2+-dependent pores in isolated mitochondria is associated with the above-described modulating effects of Pi.
Saturated α,ω-dicarboxylic acids are also capable of inducing CsA-insensitive permeabilization of the inner membrane (pore opening) of liver mitochondria. Among these acids, HDA is the most effective inducer of the pore, which is not inferior to palmitic acid in its activity [24, 26]. In experiments on model membrane systems–liposomes–it was established that in the presence of Ca2+ HDA is able to induce permeabilization of these vesicles and the release of the sulforhodamine B fluorescent probe from them, but significantly less efficiently than palmitic acid [24]. Thus, the mechanism of the action of HDA as an inducer of Ca2+-dependent pore in isolated mitochondria is not fully understood. In particular, it has not been studied to what extent this effect of HDA is associated with the above-described modulating effects of Pi.
In this work, we compared the Ca2+-dependent effects of HDA and palmitic acid on isolated liver mitochondria in the absence and presence of CsA and Pi. The obtained data are considered as evidence of significant differences in the mechanisms of action of HDA and palmitic acid as inducers of Ca2+-dependent pore in the liver mitochondria. Thus, if the Ca2+-dependent effect of palmitic acid can be considered as the formation of a lipid pore, then the effect of HDA is associated with the formation of a Ca2+-dependent pore, which is blocked by Pi in the presence of CsA.
MATERIALS AND METHODS
Mitochondria from the liver of mature male white rats weighing 210–250 g were isolated by conventional differential centrifugation with subsequent separation of endogenous fatty acids with fatty acid free BSA as described previously [27]. The isolation medium contained 250 mM sucrose, 1 mM EGTA, 5 mM MOPS–Tris (pH 7.4). The mitochondrial protein concentration was determined by the biuret method with bovine serum albumin solution used as a standard. During the experiment, the suspension of mitochondria (60–70 mg mitochondrial protein in 1 mL) was stored on ice in a narrow tube.
The change in the optical density of the mitochondrial suspension (A) was recorded at 540 nm using an Ocean Optics FLAME-T-UV-VIS spectrometer. The rate of mitochondrial swelling (Vmax = ΔA540 min–1 mg–1 protein) was determined as the change in optical density of the mitochondrial suspension during the first minute of swelling. The permeability of the mitochondrial inner membrane for Ca2+ was determined using a Ca2+ selective electrode, as in [24]. The transmembrane electric potential (Δψ) on the inner mitochondrial membrane was estimated by the distribution of tetraphenylphosphonium (TPP+) across the mitochondrial membrane, which concentration was measured with a TPP+-sensitive electrode [28]. The changes in TPP+ and Ca2+ concentrations in the medium were recorded simultaneously in a 1.2-mL cuvette at 25°C, at constant stirring and aeration using the multichannel electrometrical system Record-4usb. The incubation medium contained 200 mM sucrose, 20 mM KCl, 20 μM EGTA, 5 mM succinic acid, and 10 mM Mops–Tris (pH 7.4). In the course of the experiments, rotenone (2 μM) and, if necessary, TPP+ (1 μM) and CsA (1 μM) were added to the cell or cuvette immediately after mitochondria. All other additions were performed as indicated in the figures. In control experiments, the solvents were added to mitochondria in the same amount as in the supplement that contains the test substance. In all cases, the solvents had no effect on the mitochondrial parameters.
HDA, palmitic acid, succinic acid, CsA, bovine serum albumin (fraction V), MOPS, tris, alamethicin, Na3VO4, mersalyl, n-ethylmaleimide (Sigma, USA); rotenone, EGTA (Serva, Germany); sucrose, tetraphenylphosphonium chloride, KCl, (Fluka,); KH2PO4, CaCl2 (Merck, Germany) were used in this study. Stock solutions of HDA and palmitic acid (20 mM) were prepared in double-distilled ethanol.
RESULTS
In the introduction, it was already noted that one of the effective modulators of a CsA-sensitive pore is Pi, which increases the sensitivity of mitochondria to Ca2+ as an inducer of a pore and at the same time enhances the effect of CsA as a pore blocker. These effects of Pi reach close to maximum values already at a concentration of 1 mM [15]. It has been established that the formation of Ca2+-dependent CsA-sensitive and insensitive pores in the inner membrane of the liver mitochondria is accompanied by a high-amplitude swelling of these organelles incubated in a sucrose medium [19–22]. Swelling of mitochondria leads to decrease in the light passing through the suspension, and it can be recorded as a reduction in the optical density of the suspension of mitochondria [19–22].
In preliminary studies, we found that the addition of 40 µM of calcium chloride (100 nmol per 1 mg of protein) to liver mitochondria incubated without CsA and Pi does not result in organelle swelling (data not shown). However, in the presence of 1 mM Pi in the incubation medium, the addition of the same amount of calcium chloride induces mitochondrial swelling (Figs. 1a, 1c, curves 1). Under these conditions, 1 µM CsA completely blocks the effect of Pi (Figs. 1a, 1c, curves 2). In the absence of CsA, HDA induces more intense Ca2+-dependent mitochondrial swelling than Pi (Figs. 1a, 1b, curve 3). In this case, Pi practically does not affect the intensity of organelle swelling (Fig. 1a, curve 4, Fig. 1b, column 4). As shown earlier [26] and confirmed in the present work (data not shown), in the absence of Pi CsA does not affect the Ca2+-dependent mitochondrial swelling induced by HDA. However, in the case of incubation of mitochondria simultaneously with CsA and Pi, HDA does not induce the swelling of these organelles (Fig. 1a, curve 5 and Fig. 1b, column 5).
The effect of inorganic phosphate (Pi) and CsA on the kinetics and rate (Vmax) of changes in the optical density of liver mitochondria suspension induced by HDA (a, b) and palmitic acid (c, d). The numbers denote the curves obtained upon the addition of various agents: 1, Pi + Ca2+; 2, Pi + CsA + Ca2+; 3, Ca2+ + palmitic acid or Ca2+ + HDA; 4, Pi + Ca2+ + palmitic acid or Pi + Ca2+ + HDA; 5, Pi + CsA + Ca2+ + palmitic acid or Pi + CsA + Ca2+ + HDA. The conditions of the experiment and medium composition are described in the Materials and Methods section. Pi (1 mM) was added to the incubation medium before the addition of mitochondria; CsA (1 μM), immediately after the addition of the mitochondria. Other additives: 40 μM calcium chloride (Ca2+), 30 μM HDA, 30 μM palmitic acid (PA). The concentration of mitochondrial protein in the cell is 0.4 mg/mL. Here and in Figs. 3 and 4 the data from a typical experiment are shown that were obtained on one preparation of mitochondria. Similar results were obtained in two independent experiments. Diagrams show means ± SEM (n = 3).
Comparison of the above-described effects of HDA with the action of palmitic acid as a well-studied inducer of the Ca2+-dependent CsA-insensitive lipid pore [19–22] showed that, in the absence of CsA, palmitic acid, like HDA, induces more intense mitochondrial swelling than Pi (Fig. 1c, curve 3 and Fig. 1d, column 3). In this case, Pi also does not affect the intensity of mitochondrial swelling (Fig. 1c, curve 4 and Fig. 1d, column 4). However, palmitic acid, unlike HDA, is able to induce Ca2+-dependent swelling of liver mitochondria when 1 mM Pi and 1 μM CsA were simultaneously present in the incubation medium (Fig. 1c, curve 5 and Fig. 1d, column 5).
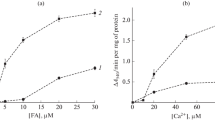
Figure 2a shows the data of a comparative study of the dependence of the initial rate of Ca2+-dependent mitochondrial swelling induced by HDA and palmitic acid, incubated with 1 μM CsA, as a function of Pi concentration. As can be seen from Fig. 2 (curve 1), Pi already at a concentration of 0.2 mM effectively blocks HDA-induced swelling of organelles. Under the same conditions, but in the case of replacement of HDA with palmitic acid Pi at a concentration of 0.2–1 mM, on the contrary, increases the rate of mitochondrial swelling (Fig. 2a, curve 2).
Dependence of the relative rate of change in optical density (V, %) of liver mitochondrial suspension incubated with Ca2+ and CsA on the concentration of inorganic phosphate (Pi) (a) and vanadate (b) in the presence of HDA (curve 1) and palmitic acid (curve 2). The experimental conditions and additions as in Fig. 1. Pi and vanadate (V) in the indicated concentrations are added to the incubation medium prior to the addition of mitochondria. The data are represented as mean ± SEM (n = 3).
The effects of Pi as a modulator of the Ca2+-dependent pore can be compared with the effects of the penetrating vanadate anion with similar properties [15, 16]. As shown in Fig. 2b (curve 1), vanadate reduces the rate of HDA/Ca2+-induced mitochondrial swelling even at a concentration of 5 mM by only 40%. At the same concentration, vanadate does not affect the palmitate/Ca2+-induced mitochondrial swelling (Fig. 2b, curve 2).
In the next series of experiments, the effect of HDA on transmembrane Ca2+ transport and Δψ in liver mitochondria was studied under the condition of their incubation with 1 mM Pi. As shown earlier [26] and confirmed in the present work (data not shown), liver mitochondria incubated without Pi and CsA in a sucrose medium are able to absorb the added Ca2+ for 2 min and retain it in the matrix for at least 12 min. In the presence of 1 mM Pi in the incubation medium, mitochondria absorb 50 μM Ca2+ in less than 1 minute and retain it in the matrix for 10 minutes (Figs. 3a, 3c, curves 1), which is accompanied by a gradual decrease in Δψ (Figs. 3b, 3d, curves 1). In this case, 1 μM CsA increases the calcium retention time in the matrix (Figs. 3a, 3c, curves 2) and prevents the decrease in Δψ (Figs. 3b, 3d, curves 2). The addition of the known channel-forming agent alamethicin (5 μg/mL) to the liver mitochondria [29] induces an intense release of Ca2+ from the matrix (Figs. 3a, 3c, curves 2) and a drop in Δψ (Figs. 3b, 3d, curves 2). In the case of incubation of mitochondria with Pi, HDA (30 μM) in the absence of CsA induces a rapid release of Ca2+ from the mitochondrial matrix (Fig. 3a, curve 3) and a complete drop in Δψ (Fig. 3b, curve 3). At the same time, in the case of incubation of mitochondria with CsA and Pi, HDA does not induce the release of Ca2+ from the matrix (Fig. 3a, curve 4) and does not affect Δψ (Fig. 3b, curve 4).
Effect HDA (a, b) and palmitic acid (c, d) on the kinetics of Ca2+ (a, c) and TPP+ (b, d) release from mitochondria incubated in the presence of Pi. The numbers denote the curves obtained upon the addition of various agents: 1, Pi + Ca2+; 2, Pi + CsA + Ca2+; 3, Pi + Ca2+ + palmitic acid or HDA; 4, Pi + CsA + Ca2+ + palmitic acid or HDA. The concentration of mitochondrial protein in the cell is 1.0 mg/mL. Additions: 1 μM CsA (not shown), 1 mM Pi (not shown), 50 μM calcium chloride (Ca2+), 30 μM HDA, 30 μM palmitic acid (PA), 5 μg/mL alamethicin (Ala).
In the presence of Pi, but without CsA, palmitic acid, like HDA, effectively induces a rapid release of Ca2+ from the mitochondrial matrix (Fig. 3c, curve 3) and a complete drop in Δψ (Fig. 3d, curve 3). However, unlike HDA, palmitic acid also effectively induces a rapid release of Ca2+ from the mitochondrial matrix (Fig. 3c, curve 4) and a complete fall in Δψ (Fig. 3d, curve 4) in the case of incubation of mitochondria with CsA and Pi.
Vanadate even at a concentration of 2 mM, i.e., higher than the Pi concentration, in the presence of CsA did not affect the ability of HDA to induce the release of Ca2+ from the matrix and the fall in Δψ (data not shown). These and the data presented in Figs. 2a, 2b show that in our conditions Pi as a blocker of HDA-induced pore cannot be replaced by penetrating anion vanadate with similar properties.
It can be assumed that the effect of Pi as a pore blocker is possible only when it is transported into the matrix with the participation of a special phosphate carrier and, therefore, can be eliminated by inhibiting this transporter. Effective inhibitors of the phosphate carrier are SH-reagents mersalyl and n-ethylmaleimide, which cause complete inhibition of the activity of this transporter in the liver mitochondria at a concentration of 10 and 170 nmol/mg protein, respectively [30]. It is known that mersalyl does not pass through the inner membrane of the liver mitochondria and interacts with the SH-group Cys41 of the phosphate carrier on its outer surface, and n-ethylmaleimide interacts with the same SH-group [31].
As was previously established [32] and confirmed in the present work (data not shown), mersalyl at a concentration of 10 nmol/mg protein suppresses the mitochondrial respiration in state 3 almost to state 4, but it only slightly inhibits the mitochondrial respiration in the case of oxidation of succinate in the presence of 50 μM 2,4-dinitrophenol. Consequently, mersalyl in this concentration completely suppresses the activity of the phosphate carrier, but has little effect on the succinate oxidase activity of mitochondria. As is shown in Fig. 4a, the addition of mersalyl to the suspension of rat liver mitochondria incubated in the presence of CsA and in the absence of Pi, reduces the effectiveness of HDA as an inducer of Ca2+-dependent organelle swelling, and also removes the inhibitory effect of Pi in case of its preliminary addition to the incubation medium. N-ethylmaleimide (200 nmol/mg protein) has a similar effect (Fig. 4b). These data suggest that the effect of Pi as a blocker of HDA/Ca2+-induced pore is eliminated in the case of incubation of mitochondria with the indicated SH-reagents that effectively act as inhibitors of the phosphate carrier.
The effect of mersalyl (a) and n-ethylmaleimide (NEM, b) on the kinetics of CsA-insensitive changes in the optical density of liver mitochondrial suspension induced by HDA in the presence and absence of Pi. The numbers denote the curves obtained upon the addition of various agents: 1, CsA + Ca2+ + HDA; 2, Pi + CsA + Ca2+ + HDA; 3, CsA + NEM or mersalyl + Ca2+ + HDA; 4, CsA + Pi + NEM or mersalyl + Ca2+ + HDA. 5 μM mersalyl or 100 μM NEM are added to the incubation medium prior to the addition of mitochondria. Other experimental conditions and additions are given in the caption to Fig. 1.
DISCUSSION
The molecule of HDA differs from the palmitic acid molecule only by the carboxyl group located at the end of the acyl chain–in the ω-position. As was shown earlier [24, 26] and confirmed in the present work (Figs. 1–3), HDA, like palmitic acid, in the liver mitochondria loaded with Ca2+ causes a high amplitude swelling of these organelles, the release of Ca2+ from the matrix and the fall Δψ, equally effectively in the absence and in the presence of CsA. It could be assumed that the mechanism of HDA action, like palmitic acid, consists in the formation of a Ca2+-dependent lipid pore in the inner membrane of mitochondria. However, HDA as an inducer of Ca2+-dependent permeabilization of liposomes is significantly inferior to palmitic acid in efficiency [24]. HDA is significantly inferior to this fatty acid and as an inductor of Ca2+-dependent permeabilization of the erythrocyte plasma membrane associated with cell lysis [33]. In the present work, it was established that the effect of HDA as an inducer of the Ca2+-dependent mitochondrial pore is blocked if the mitochondria are incubated simultaneously with CsA and Pi (Figs. 1 and 3). All this makes it unlikely that the action of HDA as an effective inducer of Ca2+-dependent permeabilization of liver mitochondria is associated with the formation of a lipid pore in the inner membrane.
In contrast to HDA, palmitic acid in Ca2+-loaded liver mitochondria incubated with Pi and CsA causes high-amplitude swelling of these organelles, the release of Ca2+ from the matrix and the Δψ drop as effectively as in the absence of these agents (Figs. 1 and 3). Therefore, Pi and CsA, in the case of their combined action, do not affect the effectiveness of palmitic acid as an inducer of Ca2+-dependent permeabilization of liver mitochondria. Obviously, this is possible in the case when palmitic acid causes the formation of a Ca2+-dependent pore in the lipid phase of the mitochondrial inner membrane, since neither CsA nor Pi affect this process. Thus, HDA and palmitic acid differ significantly in their mechanism of action as inducers of Ca2+-dependent pore. So, if the Ca2+-dependent effect of palmitic acid can be considered as the formation of a lipid pore, then the Ca2+-dependent effect of HDA is the formation of a pore that is sensitive to the combined action of Pi and CsA. Therefore, in this case Pi can be considered as a blocker of the HDA/Ca2+-induced pore.
The effect of Pi as a blocker of the HDA/Ca2+-induced pore is eliminated by incubating the mitochondria with SH-reagents mersalyl and n-ethylmaleimide, effective inhibitors of the Pi carrier. It is known that the transport of Pi into the mitochondria leads to the binding of free Ca2+ in the matrix [34]. One would assume that the effect of Pi as an inhibitor of the HDA/Ca2+-induced pore is due to a decrease in the effective concentration of free Ca2+ in the matrix. However, the fact that the blocking effect of Pi is manifested only in the presence of low concentrations of CsA, as well as the fact that Pi cannot be replaced by the penetrating vanadate anion with similar properties, makes it possible to consider this mechanism as unlikely.
It is well established that in the mitochondrial matrix CsA inhibits the binding of cyclophilin D to membrane sites [7, 35, 36]. These sites apparently are fragments of membrane proteins: ADP/ATP antiporter [36], phosphate carrier [37], F0F1-ATP synthase [7] which face the matrix. As was already noted, the existence of a regulatory site on the inner surface of the mitochondrial membrane is postulated, Pi binding to this site is accompanied by inhibition of the pore; in the absence of CsA, it is masked by cyclophilin D [7, 15]. Later it was suggested that such a regulatory site could be the site of Pi binding to F1 complex of F0F1-ATP synthase [7]. It was also suggested that CsA, inhibiting the binding of cyclophilin D to F0F1-ATP synthase on the inner surface of the inner membrane, causes unmasking of the regulatory site, and thus promotes the interaction of Pi with it [7]. Recently it was shown that genetic inactivation of cyclophilin D not only blocks the induction of pores by F0F1-ATP synthase, but also increases the efficiency of oxidative phosphorylation in mitochondria due to active assembly of ATP synthasomes [38]. Based on the foregoing, it can be assumed that the binding of Pi to this site of F0F1-ATP synthase can lead to blocking the action of HDA as an inducer of Ca2+-dependent pore in the liver mitochondria, as well as to the activation of the energy functions of organelles. However, it is also impossible to exclude the fact that the action of HDA as an inducer of Ca2+-dependent pore in liver mitochondria is performed with the participation of a phosphate carrier and/or ADP/ATP antiporter, with which cyclophilin D is able to bind. The fact that in the absence of Pi, CsA does not affect the effect of HDA as an inducer of the Ca2+-dependent mitochondrial pore suggests that binding of cyclophilin D to membrane sites is not a necessary condition for the formation of this pore. Apparently, Pi blocks the action of HDA as an inducer of a pore only if CsA inhibits the binding of cyclophilin D to membrane sites on the inner surface of the inner membrane. It is known that the effect of Pi as an inhibitor of Ca2+-dependent mitochondrial pore is unique, since penetrating anions arsenate and vanadate with similar properties do not have such an effect [15]. It was already noted above that in our conditions Pi as a blocker of the HDA-induced pore could not be replaced by a penetrating anion vanadate with similar properties.
REFERENCES
Reddy J.K., Rao M.S. 2006. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 290, 852–858.
Wanders R.J., Komen J., Kemp S. 2011. Fatty acid omega-oxidation as a rescue pathway for fatty acid oxidation disorders in humans. FEBS J. 278, 182–194.
Orellana M., Rodrigo R., Valdes E. 1998. Peroxisomal and microsomal fatty acid oxidation in liver of rats after chronic ethanol consumption. Gen. Pharmacol. 31, 817–820.
Tonsgard J.H. 1986. Serum dicarboxylic acids in patients with Reye syndrome. J. Pediatr. 109, 440–445.
Glasgow J.F., Middleton B. 2001. Reye syndrome-insights on causation and prognosis. Arch. Dis. Child. 85, 351–353.
Zorov D.B., Juhaszova M., Sollott S.J. 2014. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94, 909–950.
Bernardi P., Rasola A., Forte M., Lippe G. 2015. The mitochondrial permeability transition pore: Channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol. Rev. 95, 1111–1155.
Halestrap A.P., Davidson A.M. 1990. Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis–trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem. J. 268, 153–160.
Richardson A.P., Halestrap A.P. 2016. Quantification of active mitochondrial permeability transition pores using GNX-4975 inhibitor titrations provides insights into molecular identity. Biochem. J. 473, 1129–1140.
Bonora M., Bononi A., De Marchi E., Giorgi C., Lebiedzinska M., Marchi S., Patergnani S., Rimessi A., Suski J.M., Wojtala A., Wieckowski M.R., Kroemer G., Galluzzi L., Pinton P. 2013. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell. Cycle. 12, 674–683.
Gerle C. 2016. On the structural possibility of pore-forming mitochondrial FOF1 ATP synthase. Biochim. Biophys. Acta. 1857, 1191–1196.
Gutierrez-Aguilar M., Douglas D.L., Gibson A.K., Domeier T.L., Molkentin J.D., Baines C.P. 2014. Genetic manipulation of the cardiac mitochondrial phosphate carrier does not affect permeability transition. J. Mol. Cell. Cardiol. 72, 316–325.
Halestrap A.P. 2014. The C ring of the F1FO ATP synthase forms the mitochondrial permeability transition pore: A critical appraisal. Front. 4, 234.
Zhou W., Marinelli F., Nief C., Faraldo-Gomez J.D. 2017. Atomistic simulations indicate the c-subunit ring of the F1Fo ATP synthase is not the mitochondrial permeability transition pore. Elife. 6, e23781.
Basso E., Petronilli V., Forte M.A., Bernardi P. 2008. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J. Biol. Chem. 283, 26307–26311.
McGee A.M., Baines C.P. 2012. Phosphate is not an absolute requirement for the inhibitory effects of cyclosporin A or cyclophilin D deletion on mitochondrial permeability transition. Biochem. J. 443, 185–191.
Chavez E., Moreno-Sanchez R., Zazueta C., Rodriguez J.S., Bravo C., Reyes-Vivas H. 1997. On the protection by inorganic phosphate of calcium-induced membrane permeability transition. J. Bioenerg. Biomembr. 29, 571–577.
Bodrova M.E., Dedukhova V.I., Samartsev V.N., Mokhova E.N. 2000. Role of ADP/ATP-antiporter in fatty acid-induced uncoupling of Ca2+-loaded rat liver mitochondria. IUBMB Life. 50, 189–194.
Sultan A., Sokolove P. 2001. Palmitic acid opens a novel cyclosporin A-insensitive pore in the inner mitochondrial membrane. Arch. Biochem. Biophys. 386, 37–51.
Sultan A., Sokolove P. 2001. Free fatty acid effects on mitochondrial permeability: An overview. Arch. Biochem. Biophys. 386, 52–61.
Mironova G.D., Gritsenko E., Gateau-Roesch O., Levrat C., Agafonov A., Belosludtsev K., Prigent A., Muntean D., Dubois M., Ovize M. 2004. Formation of palmitic acid/Ca2+ complexes in the mitochondrial membrane: A possible role in the cyclosporin-insensitive permeability transition. J. Bioenerg. Biomembr. 36, 171–178.
Belosludtsev K.N., Belosludtseva N.V., Agafonov A.V., Astashev M.E., Kazakov A.S., Saris N.E., Mironova G.D. 2014. Ca2+-dependent permeabilization of mitochondria and liposomes by palmitic and oleic acids: A comparative study. Biochim. Biophys. Acta. 1838, 2600–2606.
Belosludtsev K.N., Trudovishnikov A.S., Beloslud-tseva N.V., Agafonov A.V., Mironova G.D. 2010. Palmitic acid induces the opening of a Ca2+-dependent pore in the plasma membrane of red blood cells: Possible role of the pore in erythrocyte lysis. J. Membr. Biol. 237, 13–19.
Dubinin M.V., Samartsev V.N., Astashev M.E., Kazakov A.S., Belosludtsev K.N. 2014. A permeability transition in liver mitochondria and liposomes induced by α,ω-dioic acids and Ca2+. Eur. Biophys. J. 43, 565–572.
Agafonov A., Gritsenko E., Belosludtsev K., Kovalev A., Gateau-Roesch O., Saris N.-E.L., Mironova G.D. 2003. A permeability transition in liposomes induced by the formation of Ca2+/palmitic acid complexes. Biochim. Biophys. Acta. 1609, 153–160.
Dubinin M.V., Adakeeva S.I., Samartsev V.N. 2013. Long chain α,ω-dioic acids as inducers of cyclosporine A-insensitive nonspecific permeability of the inner membrane of liver mitochondria loaded with calcium or strontium ions. Biochemistry (Moscow). 78, 412–417.
Samartsev V.N., Smirnov A.V., Zeldi I.P., Markova O.V., Mokhova E.N., Skulachev V.P. 1997. Involved of aspartate/glutamate antiporter in fatty acid-induced uncoupling of liver mitochondria. Biochim. Biophys. Acta. 1339, 251–257.
Kamo N., Muratsugu M., Hondoh R., Kobatake Y. 1979. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenylphosphonium and reationship between proton electrochemical potential and phosphorylation potential in steady state. J. Membr. Biol. 49, 105–121.
Gostimskaya I.S., Grivennikova V.G., Zharova T.V., Bakeeva L.E., Vinogradov A.D. 2003. In situ assay of the intramitochondrial enzymes: Use of alamethicin for permeabilization of mitochondria. Anal. Biochem. 313, 46–52.
Ligeti E., Brandolin G., Dupont Y., Vignais P.V. 1985. Kinetic of Pi–Pi exchange in rat liver mitochondria. Rapid filtration experiments in the millisecond time range. Biochemistry. 24, 4423–4428.
Ferreira G.C., Pratt R.D., Pedersen P.L. 1990. Mitochondrial proton/phosphate transporter. An antibody directed against the COOH terminus and proteolytic cleavage experiments new insights about its membrane topology. J. Biol. Chem. 265, 21 202–21 206.
Samartsev V.N., Chezganova S.A., Polishchuk L.S., Paydyganov A.P., Vidyakina O.V., Zeldi I.P. 2003. Temperature dependence of rat liver mitochondrial respiration with uncoupling of oxidative phosphorylation by fatty acids. Influence of inorganic phosphate. Biochemistry (Moscow). 68, 618–626.
Dubinin M.V., Stepanova A.E., Scherbakov K.A., Samartsev V.N., Belosludtsev K.N. 2016. Ca2+-dependent aggregation and permeabilization of erythrocytes by ω-hydroxypalmitic and α,ω-hexadecandioic acids. Biophysics. 61, 901–905.
Chalmers S., Nicholls D.G. 2003. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 278, 19062–19070.
Nicolli A., Basso E., Petronilli V., Wenger R.M., Bernardi P. 1996. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, a cyclosporin A-sensitive channel. J. Biol. Chem. 271, 2185–2192.
Woodfield K., Ruck A., Brdiczka D., Halestrap A.P. 1998. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem. J. 336, 287–290.
Leung A.W., Varanyuwatana P., Halestrap A.P. 2008. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J. Biol. Chem. 283, 26 312–26 323.
Beutner G., Alanzalon R.E., Porter G.A Jr. 2017. Cyclophilin D regulates the dynamic assembly of mitochondrial ATP synthase into synthasomes. Sci. Rep. 7, 14 488.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Education and Science of the Russian Federation (project nos. 17.4999.2017/8.9 and 6.5170.2017/8.9), by the Russian Foundation for Basic Research and Moscow Region (project no. 17-44-500584), and by a grant from the Mari State University (project no. 2018-03b).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by M. Dubinin
Rights and permissions
About this article
Cite this article
Dubinin, M.V., Samartsev, V.N., Starinets, V.S. et al. Induction of the Ca2+-Dependent Permeability Transition in Liver Mitochondria by α,ω-Hexadecanedioic Acid is Blocked by Inorganic Phosphate in the Presence of Cyclosporin A. Biochem. Moscow Suppl. Ser. A 13, 58–66 (2019). https://doi.org/10.1134/S1990747818040050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747818040050