Abstract—
Supraependymal plexus of the brain ventricles is one of the most mysterious structural formations in the central nervous system. Since both the topography of supraependymal elements and their functional significance remain unclear, the aim of this study was to study the distribution of supraependymal structures within the ventricular system of the rat brain with synaptic function associated marker, synaptophysin. Serial sections of Wistar rats forebrain (4–6 months, n = 6) were examined using immunohistochemical detection of synaptophysin and tyrosine hydroxylase. It was demonstrated that supraependymal structures form small discrete clusters on the apical surface of ependymocytes, which indicates synaptic contacts. Although catecholaminergic nerve fibers were present on the ventricular surface in all studied zones, it seems that these nerve fibers may not always contain synaptophysin. Thus, it is supposed that the functional purpose of the supraependymal nerve plexus depends on its localization and can be associated both with the regulation of the functional status of ependymal cells and the formation of the cerebrospinal fluid composition and with the formation of interneuronal synaptic communications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Supraependymal structures in the brain ventricles were first discovered in the 1970s by ultrastructural studies of Chan-Palay, 1976; Cupédo, 1977; Richards et al., 1981. They can include supraependymal glial and nerve cells, as well as epiplexus macrophages (Kolmer cells) (Cupédo, 1977). To some extent, supraependymal elements are represented by nerve plexuses that are formed by fibers passing along the surface of the ependymal layer. It has been supposed that these fibers are mainly formed by the processes of supraependymal neurons (Martínez and de Weerd, 1977); however, further studies demonstrated that these elements can also be formed by fibers of serotonergic neurons of the raphe nuclei (Cupédo and de Weerd, 1980). There exists an opinion that the nerve plexus located on the ependymal surface of the lateral ventricles consists of underlying dopaminergic neurons passing through the ependymal layer (Troshev et al., 2022). Since the structural study of supraependymal structures has traditionally been carried out using methods of electron microscopy that are time-consuming and difficult to standardize, it is currently unclear how common these elements are in the ventricles of the brain; estimated neurotransmitters and functional significance of supraependymal fibers remain unclear as well. Recently, an additional emphasis has been placed on the endocrine functions of the brain (Ugryumov, 2009). Specifically, the presence of cerebrospinal fluid-contacting neurons in the brain araise the question of the possible involvement of supraependymal elements in endocrine regulation. In this regard, the estimation of not only their structural, but also functional, components is acquires special relevance.
The use of more accessible methods of light microscopy in the study of the supraependymal fibers distribution and its possible involvement in synaptic transmission can be carried out using immunoselective staining, that allows marking the sites of synaptic activity. Currently, several proteins associated with synaptic terminals are known. A presynaptic vesicular glycoprotein synaptophysin is one such protein (Janz et al., 1999). Synaptophysin is associated with the membrane of small synaptic vesicles and thus reveals the synaptic structures of the CNS (regardless of which neurotransmitter they contain). This property causes the widespread use of synaptophysin as a marker of synaptic plasticity and integrity in studies concerning both the central and peripheral nervous systems (Calhoun et al., 1996; Kolos et al., 2015). Therefore, it seems that a highly specific synaptophysin immunostaining will allow us to estimate the distribution and the functional significance of the supraependymal brain elements using a simpler method of sample preparation rather than electron microscopy.
Thus, the aim of the research was to study the distribution of supraependymal structures in the cerebral ventricular system of the brain in rat using a functional immunohistochemical marker (synaptophysin).
MATERIALS AND METHODS
Frontal sections of the brain of adult male Wistar rats (4–6 months, n = 6) were used as a material for the study. All procedures involving animals were carried out in accordance with the guidelines established by the Directive 86/609/EEC on the protection of Animals used for Experimental and other scientific purposes (Strasbourg, 1986) and the “Rules of Good Laboratory Practice” (order no. 199n dated April 1, 2016, of the Ministry of Health of Russia). The study was approved by the Local Ethics Committee of the Institute of Experimental Medicine (conclusion no. 2/22 of April 4, 2022). The material was fixed in zinc–ethanol–formaldehyde (Korzhevskii et al., 2015) and embedded in paraffin according to the generally accepted method. Five-micrometer-thick sections were mounted onto “Superfrost Ultra Plus” adhesive slides (Menzel Gläser, Germany). After a standard procedure of deparaffinization and rehydration, the sections were exposed to heat-induced epitope retrieval in a water solution of sodium thiosulfate (patent no. RU 2719163 C1) for 22 min. The inhibition of endogenous peroxidase was carried out by treating the sections with a 3% water solution of hydrogen peroxide, while blocking of nonspecific antigen binding sites was carried out with a blocking solution (Protein Block, Spring Bioscience, United States) for 10 min. The synaptic structures on the sections were detected using mouse monoclonal antibodies (clone SY38, ab8049, Abcam, United Kingdom) in a dilution of 1 : 60 and rabbit polyclonal antibodies (Ready-To-Use, MON-RTU1195, Monosan, Netherlands) to synaptophysin. Rabbit polyclonal antibodies to tyrosine hydroxylase (TH) (ab112, Abcam, United Kingdom) in a dilution of 1 : 1000 were used to detect catecholaminergic structures. An UltraVision Quanto HRP DAB Detection System kit (TL-060-QHL, Thermo Fisher Scientific, United States) was used as a secondary reagent. To prevent cross linking of secondary reagents with the rat’s own immunoglobulins, serum obtained from the blood of Wistar rats kept in the vivarium of the Institute of Experimental Medicine was added to the solution of secondary antibodies to a final solution concentration of 0.5%. To visualize the reaction product, 3'3-diaminobenzidine chromogen from the DAB+ kit (Agilent, United States) was used. Some of the sections were counterstained with alum hematoxylin. The obtained slides were analyzed using a Leica DM750 microscope (Germany) and photographed with an ICC50 digital camera (Leica, Germany). As a positive control for synaptophysin immunohistochemistry rat cerebellar cortex was used. Its granular layer contains a multiple large axonal terminals of glutamatergic mossy fiber afferents forming cerebellar glomeruli (Hámori and Somogyi, 1983). Sections of the rat diencephalon at the level of the substantia nigra were used as a positive control for the tyrosine hydroxylase immunohistochemistry (Rabey and Hefti, 1990). To set up a negative control of antibodies, an Antibody Diluent (Spring Bioscience, United States) was applied to the sections instead of the primary antibody solution.
RESULTS
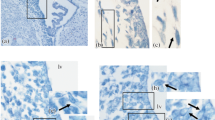
As a result of immunohistochemical reaction, synaptophysin-containing structures are clearly identified on the brain preparations. Synaptophysin-positive supraependymal elements are present on the surface of ependymal cells in the form of chains of rounded isolated granules (Fig. 1). Further analysis using high microscope magnification revealed an uneven distribution of synaptophysin-positive structures along the surface of the brain ventricles was revealed. Synaptophysin was detected in the region of lateral ventricles on the apical surface of ependymocytes of the medial and dorsal walls, but was rarely detected in the lower wall of the third ventricle in the interventricular foramen of Monro. A high density of synaptic structures was observed near the lateral walls and the bottom of the third ventricle in the dorsal hippocampal and habenular regions, but not in the infundibular recess. Subsequently, strong synaptophysin-positive reaction persists in the supraependymal structures of the dorsal part of the brain throughout the third ventricle and almost disappears in the cerebral aqueduct.
Supraependymal fibers in the brain ventricles. (a) Schemes of brain sections at the level of the studied areas; (b–i) series of successive sections showing the distribution of (b, d, f, h) synaptophysin and (c, e, g, i) tyrosine hydroxylase in different structures. (b, c) Dorsal wall of the lateral ventricle; (d, e) third ventricle, rostral part; (f, g) third ventricle, habenular region; and (h, i) cerebral aqueduct. The arrows indicate supraependymal elements; the asterisk indicates the cavity of the brain ventricles. Plan-Apochromat 100×/1.25 Oil immersion objective. Scale bars, 20 µm.
In addition to synaptophysin immunohistochemisrty, an tyrosine hydroxylase immunostaining was carried out, which allows us to visualize catecholaminergic fibers in their entire length. It was demonstrated that TH-containing fibers are present on the surface of ependymocytes in all studied areas (Figs. 1c, 1e, 1g, 1i). TH-immunopositive elements form both isolated rounded or spherical granules and nonextended fibers with varicosities. Their distribution density seems to be higher than the distribution of synaptophysin-containing structures, and the most intensive reaction was observed on the surface of the lower wall of the third ventricle in the interventricular foramen, and lateral walls wall of the third ventricle in the habenular region. Moreover, the spatial distribution of supraependymal catecholaminergic fibers often coincided with the presence of a subependymal TH-positive plexus in the underlying nervous tissue. This is typical for the lateral ventricles and adjacent white matter of the corpus callosum, habenula, and the infundibular recess of hypothalamus.
DISCUSSION
Despite numerous references in the literature, information about the supraependymal elements is currently fragmentary and mainly dates to the 1980s; therefore, it needs to be carefully validated and systematized. There is no consensus about the distribution, origins, and functional purpose of the supraependymal nerve plexus. This gap is largely due to the extreme specificity of the object of study and the peculiarities of its localization and detection on histological slides.
A methodical approach using the presynaptic protein synaptophysin immunostaining allowed us to visualize an intensive supraependymal innervation of the walls of lateral and third ventricles of the brain. It was demonstrated that the synaptic supraependymal structures form small discrete clusters on the apical surface of ependymocytes, which appeared to be sites of synaptic transmission. This is supported by ultrastructural studies demonstrating the possibility of formation of asymmetric (Gray type I) synaptic contacts with the bodies and processes of ependymal cells by supraependymal fibers (Møllgård and Wiklund, 1979; Haemmerle et al., 2015). In addition, the presence of synaptophysin in microvesicles of neuroendocrine cells was proven (Navone et al., 1986), which suggests that the supraependymal elements have a neurosecretory nature. In this context, the data of A.R. Murtazina et al. (2021), according to which monoamines present in cerebral spinal fluid (CSF) have a predominantly neuronal origin, are noteworthy. Based on the above literature data, as well as our own results, it is possible to draw the conclusion that supraependymal plexus may act as a potential source of monoamines (particularly, catecholamines) contained in CSF.
Our results showed that lateral ventricles and dorsal zone of the third ventricle of the brain are distinguished by the ubiquitous distribution of supraependymal synaptic elements. Notably, the synaptic structures of the lining of lateral ventricles are mainly concentrated in the area of the medial and dorsal, but not lateral wall. Taking into account a high relevance of ensuring the comprehensive regulation of the neurogenic niche (subventricular zone (SVZ)), one could expect astrong synaptophysin-positive reaction in this area. This is indicated by earlier ultrastructural studies that detected close synaptic contacts between varicose extensions of supraependymal serotonergic axons and apical processes of the niche cells (Tong et al., 2014), as well as by the observations of D.V. Troshev et al., who confirmed the presence of supraependymal catecholaminergic processes near the SVZ (Troshev et al., 2022). However, our data demonstrate that there is no typical synaptic innervation of the SVZ from the ventricles. According to this observation, it can be concluded that the control of the neurogenic niche is not the main functional task of the supraependymal plexus of lateral ventricles.
Another noteworthy finding came from a comparison of successive sections stained for synaptophysin and TH. It was found that the presence of nerve fibers on the surface of the ventricles is not always associated with the synaptophysin content in them. This finding suggests that such fibers lack synaptic structures. Thus, the TH-positive reaction in the area of the infundibular recess and cerebral aqueduct allows to identify the supraependymal catecholaminergic fibers that lack synaptophysin. Such specificity of the detection of two different antigens distrubution gives grounds to conclude that TH-immunopositive supraependymal could be fibers functionally inactive in some studied areas, and apparently is responsible for nerve fiber transport.
However, when considering the area of the median eminence, the structural and functional features of this area should be also taken into account. The lining of third ventricle floor is formed by tanicytes, which determines the structural and functional features of this zone (Page, 2006). It is known that a unique pattern of expression of the cell contact proteins is typical for tanycytes of the median eminence, which determines their unique properties as structural components of the brain barrier system. Particularly, it was demonstrated that α2-tanycytes exhibit a disorganized (diffuse) expression pattern of tight junction proteins which allows an exchange of molecules between the cerebrospinal fluid and underlying nervous tissue through tancytes bodies (Mullier et al., 2010). When studying the distribution of gap junction proteins, it was found that a connexin 43 protein is present in the apical part and peduncles of tanycytes, which presumably allows these cells to form hemichannels not only for the transport of small molecules into the cerebrospinal fluid (CSF), but also for bidirectional regulated transport of molecules between the CSF and blood (Sufieva et al., 2019). In this case, the median eminence area may not require additional remote neuroendocrine regulation of the supraependymal fibers.
Based on the above, it is possible to put forward three hypotheses concerning the possible function of the supraependymal plexus. First, neural processes can reach target cells not along the conduction pathways of the nervous tissue, but along the walls of the brain ventricles, and the role of supraependymal fibers in this case is to transport biologically active molecules and neurohormones. Second, supraependymal structures can affect the composition of the CSF. Third, they can provide a paracrine regulation of the function of ependymal cells. At the same time, the role of supraependymal elements is obviously region-specific.
Thus, the research conducted demonstrated that synaptophysin may be used for immunohistochemical visualization of supraependymal structures of the rat brain. Its presence in the supraependymal fibers indicates that the detected structures have a functional activity and are able to form interneuronal synaptic connections or release a neurotransmitter in the CSF, performing an endocrine regulatory function.
REFERENCES
Calhoun, M.E., Jucker, M., Martin, L.J., Thinakaran, G., Price, D.L., and Mouton, P.R., Comparative evaluation of synaptophysin-based methods for quantification of synapses, J. Neurocytol., 1996, vol. 25, p. 821. https://doi.org/10.1007/BF02284844
Chan-Palay, V., Serotonin axons in the supra- and subependymal plexuses and in the leptomeninges; their roles in local alterations of cerebrospinal fluid and vasomotor activity, Brain Res., 1976, vol. 102, p. 103. https://doi.org/10.1016/0006-8993(76)90578-3
Cupédo, R.N.J., The surface ultrastructure of the habenular complex of the rat, Anat. Embryol., 1977, vol. 152, p. 43. https://doi.org/10.1007/BF00341434
Cupédo, R.N.J. and de Weerd, H., Serotonergic intraventricular axons in the habenular region. Phagocytosis after induced degeneration, Anat. Embryol., 1980, vol. 158, p. 213. https://doi.org/10.1007/BF00315907
Haemmerle, C.A., Nogueira, M.I., and Watanabe, I.S., The neural elements in the lining of the ventricular-subventricular zone: making an old story new by high-resolution scanning electron microscopy, Front. Neuroanat., 2015, vol. 9. https://doi.org/10.3389/FNANA.2015.00134
Hámori, J. and Somogyi, J., Differentiation of cerebellar mossy fiber synapses in the rat: a quantitative electron microscope study, J. Comp. Neurol., 1983, vol. 220, p. 365. https://doi.org/10.1002/CNE.902200402
Janz, R., Südhof, T.C., Hammer, R.E., Unni, V., Siegelbaum, S.A., and Bolshakov, V.Y., Essential roles in synaptic plasticity for synaptogyrin I and synaptophysin I, Neuron, 1999, vol. 24, p. 687. https://doi.org/10.1016/S0896-6273(00)81122-8
Kolos, E.A., Grigoriyev, I.P., and Korzhevskiy, D.E., A synaptic marker synaptophysin, Morphologija, 2015, vol. 147, no. 1, p. 78.
Korzhevskii, D.E., Sukhorukova, E.G., Kirik, O.V., and Grigorev, I.P., Immunohistochemical demonstration of specific antigens in the human brain fixed in zinc–ethanol–formaldehyde, Eur. J. Histochem., 2015, vol. 59, p. 5. https://doi.org/10.4081/EJH.2015.2530
Martínez, P.M. and de Weerd, H., The fine structure of the ependymal surface of the recessus infundibularis in the rat, Anat. Embryol., 1977, vol. 151, p. 241. https://doi.org/10.1007/BF00318929
Møllgård, K. and Wiklund, L., Serotoninergic synapses on ependymal and hypendymal cells of the rat subcommissural organ, J. Neurocytol., 1979, vol. 8, p. 445. https://doi.org/10.1007/BF01214802
Mullier, A., Bouret, S.G., Prevot, V., and Dehouck, B., Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain, J. Comp. Neurol., 2010, vol. 518, p. 943. https://doi.org/10.1002/CNE.22273
Murtazina, A.R., Bondarenko, N.S., Pronina, T.S., Chandran, K.I., Bogdanov, V.V., Dilmukhametova, L.K., and Ugrumov, M.V., A comparative analysis of CSF and the blood levels of monoamines as neurohormones in rats during ontogenesis, Acta Naturae, 2021, vol. 13, no. 4, p. 89. https://doi.org/10.32607/actanaturae.11516
Navone, F., Jahn, R., Di Gioia, G., Stukenbrok, H., Greengard, P., and De Camilli, P., Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells, J. Cell Biol., 1986, vol. 103, p. 2511. https://doi.org/10.1083/JCB.103.6.2511
Page, R.B., Anatomy of the hypothalamo-hypophysial complex, in Physiology of Reproduction, Academic Press, 2006.
Rabey, J.M. and Hefti, F., Neuromelanin synthesis in rat and human substantia nigra, J. Neural Transm.: Parkinson’s Dis. Dementia Sect., 1990, vol. 2, p. 1. https://doi.org/10.1007/BF02251241
Richards, J.G., Lorez, H.P., Colombo, V.E., Guggenheim, R., Kiss, D., and Wu, J.Y., Demonstration of supra-ependymal 5-HT nerve fibres in human brain and their immunohistochemical identification in rat brain, J. Physiol. (Paris), 1981, vol. 77, p. 219.
Sufieva, D.A., Kirik, O.V., and Korzhevskii, D.E., Astrocyte markers in the tanycytes of the third brain ventricle in postnatal development and aging in rats, Russ. J. Dev. Biol., 2019, vol. 50, p. 146. https://doi.org/10.1134/S1062360419030068
Tong, C.K., Chen, J., Cebrián-Silla, A., Mirzadeh, Z., Obernier, K., Guinto, C.D., Tecott, L.H., García-Verdugo, J.M., Kriegstein, A., and Alvarez-Buylla, A., Axonal control of the adult neural stem cell niche, Cell Stem Cell, 2014, vol. 14, p. 500. https://doi.org/10.1016/J.STEM.2014.01.014
Troshev, D., Bannikova, A., Blokhin, V., Kolacheva, A., Pronina, T., and Ugrumov, M., Striatal neurons partially expressing a dopaminergic phenotype: functional significance and regulation, Int. J. Mol. Sci., 2022, vol. 23. https://doi.org/10.3390/IJMS231911054/S1
Ugryumov, M.V., Endocrine functions of brain in adult and developing mammals, Russ. J. Dev. Biol., 2009, vol. 40, no. 1, p. 14.
Funding
This work was carried out within the framework of a state order to the Institute of Experimental Medicine.
Author information
Authors and Affiliations
Contributions
V.A. Razenkova: performing immunohistochemical staining, literature analysis, interpretation of the results, working with the illustrations, writing the article text. O.V. Kirik: design of the study planning, collection of biological material and paraffin embedding, photographing and analysis of preparations, editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international guidelines for the care and use of animals were followed. The study was approved by the Local Ethics Committee of the Institute of Experimental Medicine (conclusion no. 2/22 dated April 6, 2022).
Additional information
Translated by A. Barkhash
Translated by I. Fridlyanskaya
Abbreviations: SVZ—subventricular zone; TH—tyrosine hydroxylase; CSF—cerebrospinal fluid.
Rights and permissions
About this article
Cite this article
Razenkova, V.A., Kirik, O.V. Synaptophysin Expression by Supraependymal Structures of Rat Brain. Cell Tiss. Biol. 17, 517–521 (2023). https://doi.org/10.1134/S1990519X23050115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X23050115