Abstract
Stress in the early period of life is an important factor predisposing to the development of pathology of the nervous system in animals and human beings in the late period of ontogenesis. We modeled early-life stress to assess the activation of the piriform cortex upon presentation of olfactory stimuli to experimental animals (CD1 mice of an age of 60 days and 10 months of postnatal development) and to evaluate marker expression of neurons with prolonged immaturity involved in plasticity processes of the adult brain and its recovery after injury. It has been established that early life stress reduces the number of immature neurons with DCX+PSA-NCAM+ phenotype in the piriform cortex and the response to olfactory-memory induction in the late period after stress. At the age of 60 days (P60), olfactory stimulation reduced sensitivity to unpleasant stimuli and stimulated the processes of short-term memory, while this effect is less pronounced at the age of 10 months. The results obtained indicate a possible contribution of immature neurons of the piriform cortex to the mechanisms of aberrant neuroplasticity in the late period of ontogenesis after early life stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Early life stress (ELS) is a phenomenon underlying long-term changes in brain plasticity caused by exposure to stress factors acting prenatally or in early postnatal ontogenesis (Uspenskaya et al., 2015; Malinovskaya et al., 2018; Fogelman and Canli, 2019; Kronman et al., 2021). Various prenatal or early postnatal stimuli can trigger ELS-specific mechanisms, such as hypoxia, xenobiotics, and disruption of social interactions. It affects many physiological mechanisms resulting in impaired brain development, neurological deficits, and increased susceptibility to neurodegeneration in later periods of life. This is known as the “early programming phenomenon,” or the priming of the developing brain to pathological conditions due to prenatal, intranatal, or early postnatal stress (Huang, 2014). Stress response is always associated with hyperactivation of the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system, triggering pro-inflammatory mechanisms, changes in the epigenetic regulation of gene expression that affect the normal development of brain cells and their metabolism (Salmina et al., 2021).
Recently, the interest of researchers has increased in studying the contribution of non-newly generated immature neurons (nng-IN) localized in regions of the mature brain atypical for neurogenesis (in particular, in the cortex) to the processes of neuroplasticity in normal and pathology. Thus, it is known that nng-IN has a number of important and poorly studied characteristics, such as expression of markers of immature neurons (in the period of embryogenesis), in particular, the co-expression of doublecortin proteins (DCX) and polysialylated molecule of nerve-cell adhesion (PSA-NCAM), which corresponds to the phenomenon of “arrested development”; stay in a microenvironment that does not correspond to that of immature cells (for example, formed in the neurogenic niches of the brain), which is probably provided by the astroglial cells surrounding them; lack of proliferative activity and the ability to differentiate to mature postmitotic neurons upon external signals; and close association with the vascular component of the proneurogenic microenvironment (Gómez-Climent et al., 2008, 2011; Piumatti et al., 2017). It has been shown that, during aging, some nng-IN is transformed into glutamatergic excitatory neurons (Rotheneichner et al., 2018) or into GABAergic interneurons (Benedetti et al., 2020). However, it remained unclear whether this process is also controlled by external stimuli (for example, teaching) or whether it is spontaneously realized and regulated by such neurotransmitters as glutamate and dopamine (Coviello et al., 2020).
It is very likely that the contribution of nng-IN to the processes of brain plasticity can be changed in pathological conditions associated with a developmental disorder, for example, after past ELS. This population of cells can probably be considered as the target for controlling the mechanisms of neurogenesis occurring in “nonclassical” neurogenic niches. For example, the differentiation potential of nng-IN can be controlled by suppressing PSA-NCAM expression or by modulating adrenergic mechanisms in the piriform cortex (Vadodaria et al., 2017; La Rosa et al., 2020; Bonfanti and Seki, 2021). Taking into account the contribution of postnatal neurogenesis in the “classical” neurogenic niches (subventricular and subgranular zones), the brain of an adult organism should have a very significant resource for enhancing neurogenesis and renewal of the mature neuron population when this is necessary both under physiological (learning, memory consolidation) and pathological conditions and, in particular, to prevent the development of excitation–inhibition imbalance caused by significant changes in neuron populations (Berdugo-Vega et al., 2020; Besnard and Sahay, 2021).
We hypothesized that, being localized in the piriform cortex (olfactory zone), nng-IN may be involved in the mechanisms of neuroplasticity associated with the perception of odors and generation of associative memory cells, while ELS is able to inhibit the activity of such neurons and their participation in mechanisms of brain plasticity later in the development of the organism, depleting the reserve of cells that are sensitive to external stimuli. Therefore, the aim of this work was to study changes in the nng-IN population during the implementation of the mechanisms of brain plasticity in animals that underwent ELS.
MATERIAL AND METHODS
Animals
Male CD1 mice (n = 150) were used. The animals were kept in cages with free access to water and food at constant temperature of 21 ± 1°C and a regular light cycle of a 12-h day and 12-h night.
We used an early life stress model (ELS): mother–cub separation, postnatal developmental stage P2–P14, and stressed daily for 3 h (Mirescu et al., 2004). The expected results by age P60 are neurogenesis disorders, social-behavior disorders, anxiety, and depressive-like symptoms. By the tenth month, signs of neurodegeneration are recorded in animals (Mirescu et al., 2004).
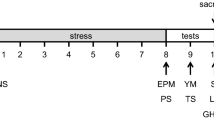
The animals were divided into two groups: one was control, consisting of intact animals aged P60 and 10 months, respectively (n = 25 in each group), and one consisted of experimental mice with ELS of the same age (P60 and 10 months, respectively, n = 25 in each). Mice from each group were subjected to olfactory stimulation (OS). The general design of the experiment is shown in Fig. 1.
Experimental scheme. ELS is early life stress. Two control groups (intact animals) and two groups of mice with ELS; age of mice 60 days of postnatal development (P60) and 10 months (n = 25 in each group). Brain tissues were sampled for immunohistochemical analysis (IHCA) 2 and 24 h and 7 days after olfactory stimulation. Five mice from each group were taken for IHCA for each time point. After 24 h, a “Fear conditioning” (contextual fear conditioning) behavioral test was performed to assess the activation of the piriform cortex at the behavioral level (n = 10 in each group).
The activation of the piriform cortex was modeled with OS according to the protocol (Schellinck et al., 2001). The OS consisted of a single sequential presentation of various odors (water, peanut butter, rat bedding) to the animal in a separate clean cage. OS was done according to the following scheme: clean water → break → peanut butter → break → peanut butter → break → rat bedding → break → rat bedding, with a stimulus duration of 2 min and break of 1 min. Brain tissues were sampled for immunohistochemical analysis (IHCA) 2, 24 h, and 7 days after OS. For each time point, 5 mice were taken from each group for IHCA.
The “Fear conditioning” behavioral test was performed after 24 h to assess the activation of the piriform cortex at the behavioral level (n = 10 for each group) (Fig. 2).
Expression of DCX+, PSA-NCAM+, and Ki-67+ markers in the piriform cortex of mice aged P60 (left) and 10 months (right). (a, b) Colocalization of DCX+ and PSA-NCAM+; (c, d) colocalization of DCX+, PSA-NCAM+, and Ki-67+; (e, f) colocalization of DCX+, PSA-NCAM+, and Ki-67−. C is intact mice (control) 2 and 24 h and 7 days after olfactory stimulation (OS). ELS is animals with early life stress before stimulation and with ELS 2 and 24 h and 7 days after OS. Mean values and their standard deviations are presented (five animals from the group, five sections from each animal, and five fields of view on the section were analyzed), with two-factor analysis of variance being used; differences between all groups are significant at p < 0.05.
IHCA
Expression of DCX, PSA-NCAM, and Ki-67 was assessed on free-floating sagittal brain sections (section thickness 50 µm) according to standard protocols. Five sections were obtained from each animal the lateral 2.9 mm–lateral 3.9 mm level according to the stereotaxic atlas of the mouse brain. The sections were washed in phosphate buffered saline (PBS) and blocked with 3% bovine serum albumin (BSA) (Sigma-Aldrich, United States) and 1% Triton X-100 (Sigma-Aldrich, United States) in PBS. After washing with the mixture of PBS and 0.2% Triton X-100, sections were incubated with primary antibodies for 20 h. The following primary antibodies were used: goat polyclonal to DCX (ab113435; Abcam, United Kingdom), mouse monoclonal to PSA-NCAM (14-9118-82; Invitrogen, United States), and rabbit polyclonal to Ki-67 (ab15580; Abcam, United Kingdom). The antibodies were used at a dilution of 1: 1000.
After incubation with primary antibodies, sections were washed five times in PBS and immersed for 1 h in the solution containing secondary antibodies: goat antimouse, Alexa Fluor 488 (ab150117; Abcam, United Kingdom); donkey antirabbit, Alexa Fluor 647 (ab150073, United Kingdom); and donkey antigoat, Alexa Fluor 555 (ab150073, Abcam, United Kingdom), all at a dilution of 1 : 1000.
As the final stage of immunohistochemical staining, 30 μL of liquid for fixing sections (70% glycerol in PBS + DAPI for staining cell nuclei) was applied in all cases and preparations were covered with coverslips,. Microscopy of sections stained for DCX, PSA-NCAM, and Ki-67 was performed on a fully automated confocal laser scanning microscope with water immersion Olympus FV10i-W (Olympus, Japan). Cells expressing DCX/PSA-NCAM and Ki67 (including simultaneously) in the piriform cortex in the pyramidal layer (second layer) were considered immunopositive. Image analysis was performed using the ImageJ software (v. 1.47, United States).
“Fear Conditioning” Test: Evaluation of the Contextual Memory of Mice
The experimental animal was placed in the apparatus (a soundproof chamber with dimensions of 31 × 24 × 21 cm and a slatted floor that was supplied with electricity to produce an electric shock). Three sessions were performed: on the first, second, and third days, respectively. Day 1 consisted of training; electric current was supplied (0.5 mA for 2 s) to the chamber in combination with a sound signal (2.8 kHz) at 120, 180, 240, 300, and 360 s from the started testing. The mouse was removed from the chamber 60 s after the last electrical current was applied. The experimental ELS groups with OS received olfactory stimulation 24 h before the start of the first session. Day 2 consisted of memory assessment; only white noise (1 kHz) was fed into the chamber. Day 3 consisted of an evaluation of short-term memory in context. The mouse was placed in the chamber for 5 min without any stimulation to assess the reproduction of behavioral responses that it remembered on the first day when stimuli were given. The freezing parameters of the animal (defensive reaction, which manifests itself as the absence of movement (except for breathing) for 0.75 s or longer) were recorded using a digital video system and ANY-maze video signal tracking software (ANY-maze, United States).
Statistical Treatment
The results of immunohistochemistry were processed using the methods of descriptive statistics, nonparametric statistics (according to the Mann–Whitney U test) when comparing two groups. When comparing three or more groups, Kruskal–Wallace H two-way analysis of variance was used. The results are presented as the mean value and its standard deviation. Differences were considered statistically significant at p < 0.05 or less.
The results of the behavioral test were processed using methods of analysis of variance (two-way Kruskal–Wallace H) followed by Bonferroni’s post-hoc test. The results are presented as the mean value and its standard error. Differences were considered statistically significant at p < 0.05 or less.
RESULTS AND DISCUSSION
Animals that have undergone ELS exhibit changes in the cell population of the piriform cortex in later period of ontogeny. In particular, we found that the number of cells with protein markers DCX and PSA-NCAM in animals with ELS at the developmental stage P60 was significantly less than in control group animals (Fig. 2a). It is noteworthy that such a change was characteristic of both DCX+PSA-NCAM+Ki67+ cells (proliferating neuroblasts, probably migrating to this region of the brain from the subventricular neurogenic niche) (Fig. 2c) and DCX+PSA-NCAM+Ki67− cells (nonproliferating immature cells that phenotypically correspond to nng-IN persisted in the cortex from the period of embryonic development) (Fig. 2e) These results are consistent with the data on ELS impact on aberrant brain maturation. This may underlie the progressive vulnerability to the development of neurodegeneration, since the DCX protein characterizes the processes of neurogenesis and marks immature cells: PSA-NCAM (marker of developing and migrating neuroblasts) and Ki-67 (marker of cell mitotic activity). It has been suggested that ELS, especially in the perinatal period, affects the maturation of brain regions responsible for cognition and emotions, enhances depressive-like behavior, and induces metabolic imbalance (Ruiz et al., 2018; Herzberg and Gunnar, 2020).
When providing OS, we found that animals with ELS did not show a changed number of DCX+PSA-NCAM+Ki67− cells characteristic of animals in the control group, namely, a decrease in their number by 2 h after the stimulus, which we interpreted as the result of induction of the final stages of differentiation and transformation into glutamatergic or GABAergic cortical neurons (Figs. 2e, 2f). In animals undergoing ELS, the number of DCX+PSA-NCAM+Ki67 cells remained stably low throughout the entire observation period after OS (after 2 and 24 h). A similar trend also applied to DCX+PSA-NCAM+Ki67+ cells (Figs. 2c, 2d). It is noteworthy that, by the seventh day after OS, the number of DCX+PSA-NCAM+Ki67+ cells increased in animals aged 10 months that had undergone ELS, which can be explained by the migration of neuroblasts from the subventricular zone (Fig. 2d).
Animals at the tenth month of postnatal development that underwent ELS were characterized by the total number of DCX+PSA-NCAM+ neurons in the piriform cortex similar to the control level (Fig. 2b), which could be interpreted as a change in the number of immature neurons in this region of the brain natural for animals of this age. Indeed, it is believed that the population of nng-IN neurons of the cortex and amygdala in the organism can decrease due to their constant conversion into glutamatergic neurons, rather than dying, during the organism’s lifetime (Rotheneichner et al., 2018; Sorrells et al., 2019).
In animals of an age of 10 months, the number of DCX+PSA-NCAM+ cells that did not exhibit mitotic activity (Ki67− cells) did not differ in control groups and after ELS; nor did it change in either group after OS (2 and 24 h and 7 days after stimulation). However, the change in the number of DCX+PSA-NCAM+Ki67+ cells in the piriform cortex was different. Initially (without stimulation), their number was the same in the control and in the group of animals with ELS, but, in the second hour after OS, their number was reduced in the control group of animals, but not in the group of animals that underwent ELS (Figs. 2, 3).
Thus, in the piriform cortex of animals that underwent ELS, in the late period of development, a reduced number of immature cells of neuronal identity, including neurons with prolonged immaturity and low responsiveness to OS presentation, are recorded. The quantitative difference in markers of immature neurons in animals of the control group is especially evident at the P60 period of postnatal development of animals, which corresponds to the end of the juvenile period of human development. Taking into account the data that it is in adolescence that a significant part of immature neurons, including, probably, nng-IN, is converted into mature glutamatergic neurons (Sorrells et al., 2019), it is not surprising that, by the tenth month of mouse development (which corresponds to the mature age of human beings) (Liu et al., 2020), such differences in the population of immature cells of the piriform cortex are actually lost.
When assessing cognitive status, it was found that olfactory stimulation reduced sensitivity to unpleasant stimuli at a young age (P60) in animals with ELS (Fig. 4), while no such effect was observed at the age of 10 months (Fig. 5). Also, the response on memorization and contextual short-term memory was more pronounced at a young age (P60) in both experimental groups.
Assessment of contextual memory (“Fear conditioning” test) in animals aged P60. (a) First day, stimulation with electric current and sound; (b) second day, stimulation with white noise; and (c) third day, context conditions without stimulation. C is control group (without OS). 24 h is preliminary OS 24 h before the test. Mean values and their standard errors are presented (Kruskal–Wallace H two-way analysis of variance followed by post-hoc Bonferroni test); * Differences are significant at p < 0.05.
Assessment of contextual memory (“Fear conditioning” test) in animals aged 10 months. (a) First day, stimulation with electric current and sound; (b) second day, stimulation with white noise; and (c) third day, context conditions without stimulation. C is control group (without OS). 24 h is preliminary OS 24 h before the test. For other explanations, see Fig. 1.
Further studies are needed to find out how (patho)physiological conditions at different stages of ontogenesis affect the population of immature neurons in the cerebral cortex, whether this is associated with metabolic changes in the processes of differentiation of immature neurons (Agostini et al., 2016; Iwata and Vanderhaeghen, 2021), as is typical, for example, for cells in the neurogenic niche of the hippocampus (Wang et al., 2020). It is logical to suppose that the development of technologies that reduce the impact of stress on the population of immature neurons in the cerebral cortex that are capable of converting into mature, functionally competent cells will help prevent or slow down the development of neurodegeneration.
Thus, as a result of the study, new data were obtained on a delayed change in the population of immature neurons in the piriform cortex after undergoing ELS. It has been established that ELS reduces the number of immature neurons with DCX+PSA-NCAM+ phenotype in the piriform cortex and their response to induced olfactory memory induction in the late period after stress (P60, 10 months). These changes affect both subpopulations of immature cells, Ki67+ and Ki67− (respectively, immature cells migrating to this region of the brain from neurogenic niches and neurons with prolonged immaturity, which have retained their presence in the cortex since the period of embryogenesis). Olfactory stimulation reduced sensitivity to unpleasant stimuli at a young age (P60) and stimulated short-term memorization processes, while this effect was less pronounced at the age of 10 months.
In general, the stimulation of olfactory memory in animals underwent ELS demonstrated a deficit of immature neurons in the piriform cortex. Since immature cortical neurons are normally capable of differentiating into mature cells under the action of stimuli that induce the mechanisms of brain plasticity, a decrease in the number of such cells in the piriform cortex in the long-term period after undergoing RPCC testifies in favor of the involvement of DCX+PSA-NCAM+ cells in the mechanisms of generation of the early programming phenomenon.
REFERENCES
Agostini, M., Romeo, F., Inoue, S., Niklison-Chirou, M.V., Elia, A.J., Dinsdale, D., Morone, N., Knight, R.A., Mak, T.W., and Melino, G., Metabolic reprogramming during neuronal differentiation, Cell Death Differ., 2016, vol. 23, p. 1502.
Benedetti, B., Dannehl, D., König, R., Coviello, S., Kreutzer, C., Zaunmair, P., Jakubecova, D., Weiger, T.M., Aigner, L., Nacher, J., Engelhardt, M., and Couillard-Després, S., Functional integration of neuronal precursors in the adult murine piriform cortex, Cereb. Cortex, 2020, vol. 30, p. 1499.
Berdugo-Vega, G., Arias-Gil, G., López-Fernández, A., Artegiani, B., Wasielewska, J.M., Lee, C.-C., Lippert, M.T., Kempermann, G., Takagaki, K., and Calegari, F., Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life, Nat. Commun., 2020, vol. 11, p. 135.
Besnard, A. and Sahay, A., Enhancing adult neurogenesis promotes contextual fear memory discrimination and activation of hippocampal-dorsolateral septal circuits, Behav. Brain Res., 2021, vol. 399, p. 112917.
Bonfanti, L. and Seki, T., The PSA-NCAM-positive “immature” neurons: an old discovery providing new vistas on brain structural plasticity, Cells, 2021, vol. 10, p. 2542.
Coviello, S., Gramuntell, Y., Castillo-Gomez, E., and Nacher, J., Effects of dopamine on the immature neurons of the adult rat piriform cortex, Front. Neurosci., 2020, vol. 14, p. 574234.
Fogelman, N. and Canli, T., Early life stress, physiology, and genetics: a review, Front. Psychol., 2019, vol. 10, p. 1668.
Gómez-Climent, M.A., Castillo-Gómez, E., Varea, E., Guirado, R., Blasco-Ibáñez, J.M., Crespo, C., Martínez-Guijarro, F.J., and Nácher, J., A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood, Cereb. Cortex, 2008, vol. 18, p. 2229.
Gómez-Climent, M.A., Guirado, R., Castillo-Gómez, E., Varea, E., Gutierrez-Mecinas, M., Gilabert-Juan, J., García-Mompó, C., Vidueira, S., Sanchez-Mataredo-na, D., Hernández, S., Blasco-Ibáñez, J.M., Crespo, C., Rutishauser, U., Schachner, M., and Nacher, J., The polysialylated form of the neural cell adhesion molecule (PSA-NCAM) is expressed in a subpopulation of mature cortical interneurons characterized by reduced structural features and connectivity, Cereb. Cortex, 2011, vol. 21, p. 1028.
Herzberg, M.P. and Gunnar, M.R., Early life stress and brain function: activity and connectivity associated with processing emotion and reward, Neuroimage, 2020, vol. 209, p. 116493.
Huang, L.-T., Early-life stress impacts the developing hippocampus and primes seizure occurrence: cellular, molecular, and epigenetic mechanisms, Front. Mol. Neurosci., 2014, vol. 7, p. 8.
Iwata, R. and Vanderhaeghen, P., Regulatory roles of mitochondria and metabolism in neurogenesis, Curr. Opin. Neurobiol., 2021, vol. 69, p. 231.
Kronman, H., Torres-Berrío, A., Sidoli, S., Issler, O., Godino, A., Ramakrishnan, A., Mews, P., Lardner, C.K., Parise, E.M., Walker, D.M., van der Zee, Y.Y., Browne, C.J., Boyce, B.F., Neve, R., Garcia, B.A., et al., Long-term behavioral and cell-type-specific molecular effects of early life stress are mediated by H3K79me2 dynamics in medium spiny neurons, Nat. Neurosci., 2021, vol. 24, p. 667.
La Rosa, C., Parolisi, R., and Bonfanti, L., Brain structural plasticity: from adult neurogenesis to immature neurons, Front. Neurosci., 2020, vol. 14, p. 75.
Liu, J.-H., Wang, Q., You, Q.-L., Li, Z.-L., Hu, N.-Y., Wang, Y., Jin, Z.-L., Li, S.-J., Li, X.-W., Yang, J.-M., Zhu, X.-H., Dai, Y.-F., Xu, J.-P., Bai, X.-C., and Gao, T.-M., Acute EPA-induced learning and memory impairment in mice is prevented by DHA, Nat. Commun., 2020, vol. 11, p. 5465.
Malinovskaya, N.A., Morgun, A.V., Lopatina, O.L., Panina, Y.A., Volkova, V.V., Gasymly, E.L., Taranushen-ko, T.E., and Salmina, A.B., Early life stress: consequences for the development of the brain, Neurosci. Behav. Physiol., 2018, vol. 48, p. 233.
Mirescu, C., Peters, J.D., and Gould, E., Early life experience alters response of adult neurogenesis to stress, Nat. Neurosci., 2004, vol. 7, p. 841.
Piumatti, M., Palazzo, O., La Rosa, C., Crociara, P., Parolisi, R., Luzzati, F., Lévy, F., and Bonfanti, L., Non-newly generated, “immature” neurons in the sheep brain are not restricted to cerebral cortex, J. Neurosci., 2017, vol. 38, p. 826.
Rotheneichner, P., Belles, M., Benedetti, B., König, R., Dannehl, D., Kreutzer, C., Zaunmair, P., Engelhardt, M., Aigner, L., Nacher, J., and Couillard-Despres, S., Cellular plasticity in the adult murine piriform cortex: continuous maturation of dormant precursors into excitatory neurons, Cereb. Cortex, 2018, vol. 28, p. 2610.
Ruiz, R., Roque, A., Pineda, E., Licona-Limón, P., Valdéz-Alarcón, J.J., and Lajud, N., Early life stress accelerates age-induced effects on neurogenesis, depression, and metabolic risk, Psychoneuroendocrinology, 2018 vol. 96, p. 203.
Salmina, A.B., Gorina, Y.V., Komleva, Y.K., Panina, Y.A., Malinovskaya, N.A., and Lopatina, O.L., Early life stress and metabolic plasticity of brain cells: impact on neurogenesis and angiogenesis, Biomedicine, 2021, vol. 9, p. 1092.
Schellinck, H.M., Forestell, C.A., and LoLordo, V.M., A simple and reliable test of olfactory learning and memory in mice, Chem. Senses, 2001, vol. 26, p. 663.
Sorrells, S.F., Paredes, M.F., Velmeshev, D., Herranz-Pérez, V., Sandoval, K., Mayer, S., Chang, E.F., Insausti, R., Kriegstein, A.R., Rubenstein, J.L., Garcia-Verdugo, J.M., Huang, E.J., and Alvarez-Buylla, A., Immature excitatory neurons develop during adolescence in the human amygdala, Nat. Commun., 2019, vol. 10, p. 2748.
Uspenskaya, Yu.A., Malinovskaya, N.A., Volkova, V.V., Panina, Yu.A., Ryabokon, R.V., Frolova, O.V., and Salmina, A.B., Development of neurological deficit after perinatal hypoxia and early life stress in laboratory animals, Sib. Med. Obozr., 2015, vol. 5, p. 49.
Vadodaria, K.C., Yanpallewar, S.U., Vadhvani, M., Toshniwal, D., Cameron Liles, L., Rommelfanger, K.S., Weinshenker, D., and Vaidya, V.A., Noradrenergic regulation of plasticity marker expression in the adult rodent piriform cortex, Neurosci. Lett., 2017, vol. 644, p. 76.
Wang, X., Liu, H., Morstein, J., Novak, A.J.E., Trauner, D., Xiong, Q., Yu, Y., and Ge, S., Metabolic tuning of inhibition regulates hippocampal neurogenesis in the adult brain, Proc. Natl. Acad. Sci. U. S. A., 2020, vol. 117, p. 25818.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 20-015-00472.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. Work with animals was carried out in compliance with the principles of humane treatment that are set out in a Directive of the European Community (2010/63/EC). The protocols of the experiments were approved by the bioethical commission for working with animals at the local ethics committee of Voyno-Yasenetsky Krasnoyarsk State Medical University.
Additional information
Translated by I. Fridlyanskaya
Abbreviations: IHCA—immunohistochemical analysis; OS—olfactory stimulation; ELS—early life stress; DCX—doublecortin; nng-IN—non-newly generated immature neurons; PBS—phosphate buffered saline; PSA-NCAM—polysialylated neural cell adhesion molecule.
Rights and permissions
About this article
Cite this article
Salmina, A.B., Uspenskaya, Y.A., Panina, Y.A. et al. Changes in the Population of Immature Neurons in the Piriform Cortex of Experimental Animals Studies in the Long-Term Period after Early Life Stress. Cell Tiss. Biol. 17, 420–427 (2023). https://doi.org/10.1134/S1990519X23040119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X23040119