Abstract
This paper is devoted to studying the effect of copper ions on the number and ratio of various subpopulations and functional properties of cells in the coelomic fluid of the starfish Asterias rubens. The experiments were carried out at the White Sea Biological Station named after N.A. Pertsov of the Faculty of Biology of Moscow State University. Starfish were exposed for 96 h to copper chloride (II) in concentrations of Cu2 + ions 0.78, 1.95, and 3.91 μM in aquariums. The number of coelomocytes was significantly increased in starfish kept in aquariums with a maximum concentration of copper ions. The distribution of cell subpopulations was also changed. The proportion of small cells increased from 9 to 15.5%, that of agranulocytes increased from 61 to 75%, and that of granulocytes decreased from 30 to 8.5%. Expression of stress-induced proteins 70 (HSC70/HSP70 ) that was determined by Western blot analysis increased in starfish in all experimental aquariums. The viability of the isolated coelomocytes estimated by the absorption of neutral red (NR) dye increased in starfish in experimental aquariums with copper ion concentrations of 0.78 and 1.95 μM as compared with the control. However, at the concentration of 3.91 μM, NR absorption drastically decreased. Taken together, it was shown that A. rubens responded to the exposure to copper (II) ions by increasing the number of circulating coelomocytes and enhancing the proportion of phagocytes subpopulation. It presumably may serve as a compensatory mechanism in response to the toxic effect of copper (II).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Pollution of the marine environment with toxic substances of anthropogenic origin significantly alters the physicochemical composition of natural waters and has a negative effect on both individual organisms and marine ecosystems in general. The problem of toxic pollutants for living organisms in a marine environment and adaptation to their effects, including at the cellular level, remains relevant. The White Sea (the inland sea in the north of the European part of Russia) is of great economic importance due to intense industrial fishing and economic activity in the coastal zone. The mining industry has a large impact on the White Sea ecosystem because of the pollution by various metals (Korshenko, 2015; Chernogaeva, 2017), of which copper is one of the most toxic. Copper is of particular interest from the point of view of toxicology, not only in connection with its high toxicity, but also because of its high biological activity. It is involved in a variety of functions in the animal body: regulation of the functional activity of certain enzymes and affecting metabolic processes, growth, and development of the whole organism.
A promising object of toxicological studies are representatives of the type Echinoderms (Echinodermata). Echinoderms are the oldest representatives of the Deuterostomia group and are of interest due to their high environmental importance and wide distribution area. Adult animals are benthic organisms distributed throughout the whole world ocean. They populate the seabed from the littoral area to almost maximum depths. Due to the fact that members of the Echinoderm type lead a largely sedentary lifestyle and are one of the final links in many food chains, they are more subjected to factors of natural (Barbaglio et al., 2012) and anthropogenic (Falugi et al., 2012) origin. Echinoderms are phylogenetically close to vertebrates and have a complex immune system that includes Toll-like receptors (Smith et al., 2010) and stress-induced proteins involved in the realization of the innate immune response (Tsan, 2009). Toxic effects of pollutants can be studied (Coteur et al., 2003; Ronning, 2005; Poromov, Smurov, 2014) on the biological material of echinoderms of different levels of organization (from subcellular to organismic),
As the object of our study, we used Asterias rubens L., a widespread euryhaline type of starfish. Cellular elements of coelomic fluid are of particular interest when studying the adaptation of this group to abiotic environmental factors. Coelomocytes play an important role in the regeneration and protective reactions of echinoderms from various types of injuries and infections. These cells circulate as part of coelomic fluid and are capable of phagocytosis of foreign particles and formation of aggregates in areas of significant body damage affecting the coelomic channels (Kozlova et al., 2006). The restoration of the coelomocytes pool when the animal is damaged occurs quite quickly. Echinoderm cells can be isolated into a primary cell culture (Sharlaimova et al., 2010) suitable for toxicity assessment of water-soluble contaminants—in particular, metal ions.
Quantitative, qualitative, and functional changes in the cellular elements of coelomic fluid were monitored under the influence of various natural and anthropogenic factors. The number of coelomocytes increases in response to injuries, infections, unfavorable conditions (Kozlova et al., 2006). Enhanced number of amoebocytes (one of the subpopulations of coelomocytes) is observed as a response to parasitic invasions; It served as the basis for considering this phenomenon as part of the inflammatory process. Injection of gram-positive bacteria into the coelomic cavity leads to an increase in the concentration of only two subpopulations of amoebocytes, which are the most active phagocytes (Coteur et al., 2004).
Studies in the Norwegian fjord Sorfjord (Coteur et al., 2003, 2004) showed that the concentration of amoebocytes and the release of reactive oxygen species (ROSs) by cells in a relatively clean and contaminated areas differed. It has also been found that the formation of micronuclei in coelomic fluid cells and the release of ROSs depends on the content of cadmium (Cd2+) and lead (Pb2+) ions in the environment (Coteur et al., 2004). Increased ROSs are a marker of apoptosis and pose a threat to the integrity of amoebocytes, as they change the activity of some macromolecules (Guzik et al., 2003).
It is also known that metal ions are able to stimulate the expression of heat shock proteins (HSP) in marine organisms (Franzellitti, Fabbri, 2005; Deane, Woo, 2005). Stress-induced heat shock proteins play an extremely important role in maintaining normal cellular homeostasis, as well as in a complex mechanism that ensures cell survival under adverse conditions (Becker, Craig, 1994; Browne et al., 2007).
In our previous work (Fedyunin, 2018), it was shown that copper was the most toxic of a number of tested metals for A. rubens L starfish. Its semilethal concentration LD50 (at which half of the population dies) is 0.98 ± 0.16 μM under exposure for 96 h. In our research, we used sublethal copper concentrations (0.78, 1.95, and 3.91 μM) that did not kill the entire experimental group of animals in the course of a 4-day exposure.
The purpose of this work was to examine the effect of copper ions in sublethal concentrations on the number of different types of cells and their ratio in various subpopulations of coelomic fluid (CG) of starfish Asterias rubens. We also studied functional indicators such as lysosomal activity and the content of stress-induced proteins of 70 kDa (HSC70/HSP70).
MATERIALS AND METHODS
Collection and Maintenance of Experimental Animals
Starfish A. rubens were collected near the White Sea Biological Station Named after N.A. Pertsov, Moscow State University, Faculty of Biology (Kandalaksha Bay of the White Sea). Only intact five-star animals with a diameter of 10–12 cm were used. Starfish were kept in glass aquariums with a volume of 20 L at a water temperature of 15 ± 2°C, salinity of 26 ‰, and a natural light cycle.
Exposure to Copper Chloride
Copper chloride solution (CuCl2 ⋅ 2H2O) (Reakhim, Russia) was added to the aquariums to the copper ion concentration of 0.78, 1.95, or 3.91 μM. Values that are multiples of environmental standards (maximum permissible concentrations) were used to calculate the experimental concentrations of metals (order of January 18, 2010, no. 20, Moscow, Russia). The water in the aquariums was changed daily by half, adding the copper chloride solution to appropriate concentrations of copper ions in the solution. Coelomic fluid (CF) of starfish A. rubens was taken after 4 days (96 h) of the experiment. The control was A. rubens starfish kept in the sea water without copper addition. Water in control aquariums was also changed daily by half.
CF Isolation
CF was removed with a 1-mL syringe from the tip of the starfish A. rubens of at least five animals from each experimental and control aquariums 4 days after the beginning of the experiment. CF of each starfish was poured into separate tubes containing an equal volume of CMFSS saline (0.5 M NaCl (LenReaktiv, Russia), 0.0026 M KCl (Helicon, Russia), 0.1 M Tris (Helikcon, Russia), pH 8.0) (Kozlova and et al., 2006), supplemented with 15 mM EDTA (Helicon, Russia) (anticoagulant), and placed on ice.
Determination of Cell Number in CF
One hundred microliters of CF were placed in separate tubes. Ten microliters of 0.6% solution of toluidine blue dye (Diaem, Russia) was added to them. It was mixed and kept for 30 min, after which the cells were counted under a microscope in a Goryaev’s chamber (Diaem, Russia). The number of coelomocytes was estimated in duplicate in five A. rubens starfish from each experimental and control aquarium.
Analysis of CF Cellular Composition
A 200-μL CF was placed in 8% formalin of equal volume (Neva-Reaktiv, Russia). Cells were fixed for 30 min in 0.5-mL plastic tubes (Eppendorf, Germany). The fixator was washed with distilled water by centrifugation. The cell pellet was resuspended in distilled water, dropped to a glass slide pretreated with polylysine (20 μg/mL), dried for 30 min, and covered with distilled water for 20 min. Next, the cells were fixed in methanol for 15 min and stained with 5a % Romanovsky–Giemsa solution (LenReactiv, Russia) for 20 min. The excess dye was washed off with distilled water, followed by dehydration in alcohols in the sequence of 70 and 96% ethanol, isobutanol, and xylene as described in (Kozlova et al., 2006) with some modifications.
The percentage of cells of various types was evaluated in at least five starfish from each experimental and control aquarium 4 days after the start of the experiment. In each CF sample, 500 cells were counted.
HSC70/HSP70 Protein Expression in Coelomocytes after Exposure to Copper. Western Blot Analysis
The cell suspension obtained from three starfish was immediately centrifuged at 1500 g for 10 min at room temperature
A cell pellet was resuspended in a lysis buffer (1% PMSF, (Diaem, Russia) and Triton X-100 and 58 mM EDTA (Helikon, Russia)) and supplemented with a mixture of protease inhibitors (2 μg/μL of antipain and leupeptin, 1 μg/μL of aprotinin and pepstatin, 1 mM benzamidine) and homogenized for 10 min on ice. Cell lysates were centrifuged at 5600 g for 10 min at 4°C. The total protein in cell lysate supernatants was determined according to the Bradford method (Bradford, 1976) using a Bradford protein assay kit (Bio-Rad, United States) according to the manufacturer’s protocol using bovine serum albumin as a standard.
Cell lysates were subjected to electrophoresis in polyacrylamide gel plates in the presence of sodium dodecyl sulfate (SDS) and β-mercaptoethanol (Helikon, Russia), and 7.5% standard cross-linked gels (2.7%) were used. Proteins were transferred on PVDF membranes at 80 V/350 mA in the buffer composed of 25 mM Tris (Helikon, Russia), 180 mM glycine (Reakhim, Russia), pH 8.3, 20% ethanol, and 0.1% SDS for 2.5 h. The filter was rinsed with 7% acetic acid and stained with 0.1% Ponceau S in 1% acetic acid. Unspecific binding was blocked by filter incubation for 1 h at room temperature in 5% skim milk solution in TSB-T buffer (20 mM Tris-HCl, pH 7.6, 165 mM NaCl, and 0.05% Tween-20 (Helikon, Russia).
As the primary antibodies, we used mouse monoclonal antibodies to heat shock proteins with a molecular mass of 70 kDa (Hsp70 McAb) (H-5147, Sigma Chemical Co., United States) at a dilution of 1 : 5000. These antibodies recognize both inducible (Hsp70) and constitutive (Hsc70) isoforms of heat shock protein. The secondary antibodies were antimouse antibodies (IgGs) conjugated to horseradish peroxidase (Amersham, United Kingdom) at a dilution of 1 : 10 000. Incubation with primary and secondary antibodies was performed in TSB-T buffer with skim milk for 1 h at room temperature. Protein bands were detected with a chemiluminescent reagent kit (Amersham, United Kingdom) and 3.3-diaminobenzidine (DAB). Membranes were scanned and the intensity of chemiluminescence was measured using the ChemiDoc system (Bio-Rad, United States) equipped with Quantity One software, version 4.2.1. Chemiluminescence intensity is presented in arbitrary units based on total analysis of the bands.
Assessment of Coelomocyte Viability
The viability of the isolated coelomocytes of the starfish A. rubens was estimated using a cytotoxic test with neutral red (NR) dye (Hauton, Smith, 2004; Kudryavtsev et al., 2016). The NR test is applied to evaluate the functional activity of lysosomes in cells vitro. NR is a cationic dye utilized for intravital staining of cells due to its selective accumulation in lysosomes by passive transport through the cell membrane. Dead cells lose their ability to accumulate neutral red in lysosomes, thereby the intensity of staining of cells characterizes their viability.
For experiments, the complete culture medium composed of RPMI-1640 medium (PanEco, Russia) with NaCl up to 24 ‰, 10% A. rubens serum, 10 mM HEPES (Diaem, Russia) and 2 mM L-glutamine and 80 μg/mL gentamicin (PanEco, Russia) was prepared. The working solution of NR (Sigma Aldrich, United States) at concentration of 40 μg /mL was prepared immediately before the experiment by adding 10 μL of the stock solution (5 mg NR in 1 mL DMSO (Helikon, Russia) to 5 mL of the complete culture medium, stored until at –20°С). One hundred microliters of cell suspension (3 × 106 cells/mL) was added to the wells of 96-well flat-bottom plate (Sarstedt, United States). Fifty microliters of NR of working solution, 50 μL of filtered seawater, or an equal volume of copper chloride solution with a concentration of copper ions of 0.78, 1.95, or 3.91 μM were added. Coelomocytes were incubated with NR for 6 h at 10°С in an atmosphere of 3% СО2.
The wells of the plate washed twice of free dye with 200 μL filtered seawater and supplemented with 100 μL fixative (1% KCl in 0.5% formalin (Neva-Reaktiv, Russia) in distilled water). NR incorporated into in the lysosomal and cytoplasmic fractions was extracted with 1% acetic acid on 50% ethanol. Spectroscopy was performed with a BioRad X-mark multichannel spectrophotometer (BioRad, United States) at a wavelength of 570 nm. The results are presented in units of optical density (OD). Viability was estimated as the ratio (%) of the OD value in the experiment to the OD value in the control.
Statistical Analysis
Statistical data processing was performed using the RStudio package (version 1.0.143). The results are presented as means value and standard deviations. One-way variance analysis (ANOVA) was used to assess the effect of copper ions at various concentrations. Intergroup comparison of quantitative indicators was assayed with the nonparametric Mann–Whitney U-test. Differences were considered statistically significant at p ≤ 0.05.
RESULTS
Water Quality Parameters and Animal Survival
The water temperature in experimental aquariums ranged from 13.4 to 15.2°C, the salinity varied from 25 to 26 units of the actual salinity, and the pH of the water ranged from 8.0 to 8.2. After A. rubens starfish exposure for 96 h to copper ions at a concentration of 1.95 μM, the death rate was 10%. It increased to 30% with copper ion concentration of 3.91 μM, (Table 1).
Total Number of Coelomocytes
The initial coelocyte number in CF was 52.7 ± 43.7 × 103 cells/μL (mean 37.7 × 103). After 4 days, the number of coelomocytes in starfish in the control aquarium decreased to 16.2 ± 30.8 × 103 cells /μL. At this period, the average number of coelomocytes in the experimental aquariums after starfish exposure to copper at maximum concentration 3.91 μM was higher than in control animals (p < 0.05). The results are shown in Table 1.
Cell Composition of CF
Microscopic examination of CF cellular composition revealed different cell morphotypes. (1) Agranulocytes (also called “phagocytic amebocytes”), cells of various shapes without granules with a well-identified nucleus, up to 7 μm in diameter, and with basophilical staining. (2) Granulocytes (also called “red spherical cells” or “red amoebocytes”) up to 7 μm in diameter, round or oval, stained eosinophilically, granules stained in a darker color, the nucleus was not identified. (3) Small cells up to 4 μm in diameter, stained blue. In starfish maintained in the control aquarium for 96 h, the amount of agranulocytes was 61%, that of granulocytes was 30%, and that of small cells was 9%. A significant difference in cell distribution was observed between individual starfish. Exposure to copper increased the proportion of small cells to 15.5% and agranulocyte number to 76%. The results are shown in Table 1.
Expression of HSC70/HSP70 Proteins
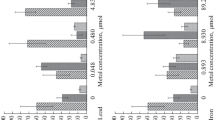
Analysis of the band intensity on immunoblots for three starfish from each experimental aquarium (Fig. 1) showed a significant increase in the expression level of stress proteins with a molecular mass of 70 kDa (HSC70/HSP70) with increasing copper concentration (ANOVA, p < 0.05).
The level of expression of HSC70/HSP70 proteins in coelomocytes of starfish A. rubens exposed to copper ions in various concentrations in experimental aquariums. Expression of HSC70/HSP70 has been measured by immunoblotting in triplicate (three animals from each aquarium) and is presented in arbitrary units. The distribution diagram here and in Fig. 2 includes the mean, standard error and standard deviation (line, rectangle border and margin error, respectively).
Viability of Starfish Coelomocytes
Cell viability was assayed by the absorption of the NR dye. Cell staining time was 6 h and was chosen in the course of preliminary experiments to adapt this technique for starfish. The results presented in Fig. 2 show that, after 6 h, NR accumulation in the lysosomes and in the cytoplasm of intact coelomocytes increased compared with copper added at a concentration of 0.78 and 1.95 μM. It drastically decreased with increased copper concentration to 3.91 μM.
DISCUSSION
Starfish A. rubens, a widespread invertebrate species in seas of northern latitudes which is part of the sea macrobenthos was used as a model object to assess the risk of environmental pollution at the subcellular level. Metals are the main component among a variety of pollutants in waters of the northeastern Atlantic Ocean. The population of starfish A. rubens lives in conditions of increased anthropogenic load, and, therefore, the question of what subcellular and cellular mechanisms are associated with the adaptation of these animals becomes relevant.
Compared with the effect of other metals studied earlier, the copper even with a slight excess of environmental standards during the long-term exposure in the marine environment is toxic and causes the death of A. rubens (Fedyunin, 2018). Despite the rapid penetration and accumulation of copper ions in the body of a starfish (Coteur et al., 2003) and their potential cytotoxicity at high concentrations in water, the number of circulating coelomocytes did not decrease in our experiments. On the contrary, after 96-h exposure. the amount of cells in all experimental animals increased by at least twice and by seven times when exposed to copper at the maximum concentration,. Copper ions probably activate cell proliferation, which is additionally confirmed by an increased number of small cells, which are presumably precursor cells (Kozlova et al., 2006).
The elevated number of agranulocytes may be associated both with an increased level of phagocytosis and participation of cells of this type in the transport of metals between tissues and to excretory organs. Mass migration of coelomocytes into the gills, digestive tract, and other detoxification organs under the influence of metals (Cd2+, Cu2+, Pb2+) was previously shown in experiments with mollusks (Soto et al., 2003). These experiments showed that mollusks can remove cadmium and copper by binding to specific proteins, called metallothioneins, as well as by their incorporation into phagocyte lysosomes.
The altered protein expression in coelomocytes of starfish A. rubens and NR dye absorption by these cells in response to copper ion exposure makes it possible to suggest that adaptation mechanisms based on the activity of heat shock proteins and increased lysosomal activity took place. Enhanced expression of proteins of the HSP70 heat shock family, revealed after 4-day exposure to copper ions at the concentration up to 1.95 μM, actually shows the response of the body and its chaperone system to stress.
However, we found that a significant increase in the total number of CF cells in response to exposure to copper ions at the maximum concentration 3.91 μM was accompanied by an increased quantity of small cells and significantly declined viability of all CF cells. Moreover, a further increase in the amount of HSC70/HSP70 protein involved in the implementation of adaptation mechanisms was not observed. Taking into account that, at a copper ion concentration above 1.95 μM, the survival rate of starfish A. rubens decreases, it can be assumed that this amount of HSC70/HSP70 is maximum and, when it is reached, the induction of protein synthesis is blocked.
Enhanced expression of stress-induced proteins was already observed with low concentrations of copper in the environment. Similar results on the expression level of stress proteins were reported for starfish A. rubens collected in areas with different levels of pollution (Coteur et al., 2004).
At high copper concentrations the cell viability is significantly reduced and increased number of coelomocytes can be a compensatory mechanism that is implemented in response to severe stress. Being toxic to coelomic fluid cells, copper is likely to adversely affect other organ systems and immune mechanisms of the animal.
The work performed showed a pronounced cytotoxic effect of copper ions on starfish exposed to its various concentrations. The toxic effect was already observed at concentrations actually encountered in the marine environment. The obtained results suggest that starfish A. rubens at the cellular and subcellular levels can be used as a bioindicator of the quality of the marine environment. Thus, animal death and toxic effects at the cellular level were observed at a copper concentration of 1.95 μM or higher; however, significant changes in cell metabolism were observed at lower concentrations.
Based on the data obtained, it can be concluded that the changes that occur in the coelomic fluid of starfish A. rubens in response to the presence of copper ions are aimed at reducing the negative effects of chemical pollution and contribute to the animal adaptation to anthropogenic effects.
REFERENCES
Barbaglio, A., Tricarico, S., Ribeiro, A., Ribeiro, C., Sugni, M., Di Benedetto, C., and Candia, D., The mechanically adaptive connective tissue of echinoderms: its potential for bio-innovation in applied technology and ecology, Mar. Environ. Res., 2012, vol. 76, p. 108.
Becker, J. and Craig, E.A., Heat-shock proteins as molecular chaperones, Eur. J. Biochem., 1994, vol. 219, p. 11.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Biochem., 1976, vol. 72, p. 248.
Browne, C.L., Swan, J.B., Rankin, E.E., Calvert, H., Griffiths, S., and Tytell, M., Extracellular heat shock protein 70 has novel functional effects on sea urchin eggs and coelomocytes, J. Exp. Biol., 2007, vol. 210, p. 1275.
Chernogaeva, G.M., Обзор состояния и загрязнения окружающей среды в Российской Федерации за 2016 год (Overview of the State and Environmental Pollution in the Russian Federation for 2016), Moscow: Rosgidromet, 2017.
Coteur, G., Corriere, N., and Dubois, P., Environmental factors influencing the immune responses of the common European starfish (Asterias rubens), Fish Shellfish Immunol., 2004, vol. 16, p. 51.
Coteur, G., Gillan, D., Joly, G., Pernet, P., and Dubois, P., Field contamination of the starfish Asterias rubens by metals. Part 2: Effects on cellular immunity, Environ. Toxicol. Chem., 2003, vol. 22, p. 2145.
Deane, E.E., and Woo, N.Y.S., Cloning and characterization of the hsp70 multigene family from silver sea bream: modulated gene expression between warm and cold temperature acclimation, Biochem. Biophys. Res. Commun., 2005, vol. 330, p. 776.
Falugi, C., Aluigi, M.G., Chiantore, M.C., Privitera, D., Ramoino, P., Gatti, M.A., Fabrizi, A., Pinsino, A., and Matranga, V., Toxicity of metal oxide nanoparticles in immune cells of the sea urchin, Mar. Environ. Res., 2012, vol. 76, p. 114.
Fedyunin, V.A., Poromov, A.A., and Smurov, A.V., The effect of metals on the survival and vitality of starfish Asterias rubens, Toksikol.Vestn., 2018, vol. 151, no. 4, p. 29.
Franzellitti, S. and Fabbri, E., Differential HSP70 gene expression in the Mediterranean mussel exposed to various stressors, Biochem. Biophys. Res. Commun., 2005, vol. 336, p. 1157.
Guzik, T.J., Korbut, R., and Adamek-Guzik, T., Nitric oxide and superoxide in inflammation and immune regulation, J. Physiol. Pharmacol., 2003, vol. 54, p. 469.
Hauton, C., and Smith, V.J., In vitro cytotoxicity of crustacean immunostimulants for lobster (Homarus gammarus) granulocytes demonstrated using the neutral red uptake assay, Fish Shellfish Immunol., 2004, vol. 17, p. 65.
Korshenko, A.N., Seawater Quality according to Hydrochemical Indicators: A Yearbook, Moscow: Nauka, 2015.
Kozlova, A.B., Petukhova, O.A., and Pinaev, G.P., The analysis of cellular elements in coelomic fluid during early regeneration of the starfish Asterias rubens L., Tsitologiia, 2006, vol. 48 No 3, p. 175.
Kudryavtsev, I., D’yachkov, I., Mogilenko, D., Sukhachev, A., and Polevshchikov, A., The functional activity of fractions of coelomocytes of the starfish Asterias rubens Linnaeus, 1758, Russ. J. Mar. Biol., 2016, vol. 42, p. 158.
Order of the Federal Agency for Fisheries of January 18, 2010, no. 20 “On Approval of Water Quality Standards for Water Bodies of Fishery Importance, Including Standards of Maximum Permissible Concentrations of Harmful Substances in the Waters of Water Bodies of Fishery Importance,” 2010.
Poromov, A.A. and Smurov, A.V., Characterization of copepod Scottomyzon gibberum Scott population on Asterias rubens L. starfishes under different anthropogenic load conditions, Moscow Univ. Biol. Sci. Bull., 2014, vol. 69, no. 2, p. 74. Ronning, B.I., Echinoderm coelomocytes as a cellular model in toxicity testing and biomonitoring. https://www.duo.uio.no/bitstream/handle/10852/11837/ ronning.pdf?sequence=3&isAllowed=y.
Sharlaimova, N.S., Pinaev, G.P., and Petukhova, O.A., Cells of coelomic liquid and cells of different tissues of sea star Asterias rubens L. isolated from intact and post-traumatic animals: behavior and proliferation under cultivation in vitro, Tsitologiia, 2010, vol. 52, no. 4, p. 317.
Smith, L.C., Ghosh, J., Buckley, K.M., Clow, L.A., Dheilly, N.M., Haug, T., Henson, J.H., Li, C., Lun, C.M., Majeske, A.J., Matranga, V., Nair, S.V., Rast, J.P., Raftos, D.A., Roth, M., Sacchi, S., Schrankel, C.S., and Stensvag, K., Echinoderm immunity, in Invertebrate Immunity, New York: Landes Biosci. Spring Sci. + Business Media, 2010, p. 260.
Soto, M., Marigomez, I., and Cancio, I., Biological aspects of metal accumulation and storage, 2013. Accessed January 25, 2018. www.ehu.es/europeanclass2003/biological_aspects_of_metal_accu.htm.
Tsan, M.F., and Gao, B., Heat shock proteins and immune system, J. Leukocyte Biol., 2009, vol. 85, p. 905.
Funding
This work was supported by the Russian Foundation for Basic Research and the city of Sevastopol as part of scientific project no. 18-44-920007 r_a.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest
Statement on the welfare of animals. Experiments with animals were carried out in accordance with the recommendations of E.A. Borisenko and Yu.K. Kissior on biomedical research using animals published at Novosibirsk State Agrarian University, Faculty of Biology and Technology, order no. 742 of 11/13/1984 approving the rules for conducting work using experimental animals; federal law no. 52-ФЗ “On the Animal World” with amendments and additions of April 24, 1995; and the interstate standard GOST (State Standard) 33044-2014 “Good Laboratory Practices” from 2015.
Additional information
Translated by I. Fridlyanskaya
Abbreviations: ROS—active oxygen species, LD50—median lethal dose, NR—neutral red, CF—coelomic fluid.
Rights and permissions
About this article
Cite this article
Fedyunin, V.A., Poromov, A.A. & Smurov, A.V. The Influence of Copper Ions on Cellular Elements of the Coelomic Fluid of Starfish Asterias Rubens L.. Cell Tiss. Biol. 14, 302–308 (2020). https://doi.org/10.1134/S1990519X20040021
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X20040021