Abstract
Entosis is a type of cell cannibalism during which one tumor cell invades another. The fate of the inner cell can vary. It can leave the entotic vacuole, divide within it, or be subjected to lysosome-mediated degradation. The aim of our work was to determine whether MCF7 (p53+) human breast adenocarcinoma cells and A431 (p53-) human epidermoid carcinoma cells can pass through the cell cycle during entosis. The percentage of entotic cells was 1.01 ± 0.37% in MCF7 culture and 0.42 ± 0.27% in A431 culture. It was shown that inner cells, as well as outer cells, can synthesize DNA (BrdU incorporation) and enter mitosis. Morphometric analysis showed polyploidization of outer cells. This process is most pronounced in the A431 (p53–) cell line. In addition, polyploid cells may be the preferred targets of invasion in this culture. In the MCF-7 cell line, the number of G1-phase entotic cells was higher, probably due to p53-mediated cell cycle arrest or the preferred cell invasion in the G1-phase. Overall, in tumors with active p53 protein expression, the entosis contribution to polyploidy and genetic instability of tumor cells is less than in p53 tumor cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Entosis is a cell cannibalism process where a tumor cell invades another (Overholtzer et al., 2007; Sharma and Dey, 2011; Yang and Li, 2012; Durgan and Florey, 2018). The cell-in-cell phenomenon correlates with malignant progression and poor prognosis for anticancer therapy (Fais, 2007; Sharma and Dey, 2011). The mechanism of entosis is being currently actively investigated. It has been demonstrated that the invasion of preliminary detached cells is a process requiring the activity of the actomyosin system and accompanied with generation of adhesive contacts between cells (Overholtzer et al., 2007). In adhered cultures, the invading cell interacts with the potential entotic (outer) cell and forms a “crater” on its surface. The entotic cell protrudes through the plasma membrane and covers the internalizing cell (Garanina et al., 2017). The cells form adhesive contacts. The invaded cell maintains viability for a time. Later, the cell undergoes lysosome-mediated degradation. Depending on the degree of degradation, the entosis process can be divided into five stages. At the first stage, both cells are connected by means of adhesive contacts and free space of entosis vacuole is not visible. The nucleus of the invading cell lacks degradation features. At the second stage, adhesive contacts between cells are partially destroyed. The third stage is characterized by total loss of contacts, visible free space of the entosis vacuole, and further nuclear degradation. At the fourth stage, the nuclear size of the invaded cell diminishes and nuclear degradation is prolonged. The chromatin of the engulfed cell is not revealed at the fifth stage. Besides degradation, mitotic division and exit of the invaded cell from the vacuole have been described (Overholtzer et al., 2007; Overholtzer and Brugge, 2008). The entotic cell is also capable of mitotic division. In this case, the entosis vacuole creates a mechanical obstacle for contractile ring formation (Krajcovic et al., 2011). The result is a polyploidy cell.
Entosis has a two-natured influence on the tumor progression. On one hand, entosis results in tumor cell death, but, on the other hand, it contributes to genetic instability.
Studies have entosis studies have almost not addressed the problem of the interrelation of these processes with the cell cycle. It is unknown how the cell cycle is regulated in entotic and invading cells, at what phase the cell may be invaded, or whether the entosis and engulfed cells display mitosis blockage or prolongation of any cell cycle phase. Answers to these questions will help to understand better the physiology of entotic cells and contribution of entosis to tumor progression.
The study was performed with human breast adenocarcinoma MCF-7 (р53+) and human epidermoid carcinoma A431 (p53–) with p53 suppressor mutation (Reiss et al., 1992). The model is suitable for estimating entosis and the invading cell’s capacity for cell cycling and polyploidization depending on p53 protein, a key regulator of the cell cycle.
MATERIALS AND METHODS
MCF-7 cells (human breast adenocarcinoma) were obtained from the Institute of Carcinogenesis, N.N. Blokhin Russian Cancer Research Center, Russia. A431 (human epidermoid carcinoma) cells were obtained from the Russian Cell Culture Collection of Vertebrates, Institute of Cytology, Russia. The cells were maintained in DMEM (PanEco) or RPMI1640 (PanEco) medium supplemented with 10% fetal bovine serum (PAA Laboratories, Austria) and 80 mg/mL gentamicin (PanEco) at 37°C and 5% CO2.
The cells were fixed with 96% ethanol at 20°С and stained with hematoxylin and eosin to calculate entosis and mitotic indices (n = 3000), as well as cell ploidy. To estimate ploidy, the area of projection of nuclei (nonentosis, entosis, and invaded cells) and entotic vacuole were measured (PO Vision Bio® Analyze, Russia). Projection areas were summarized if cell had several nuclei or micronuclei. Histograms were created using Microsoft Excel 2003. Because entosis is a rare event, standard deviation was not determined but the number of entotic events was indicated.
To reveal at what phase cells were capable of entosis, the cells were arrested at various cell cycle phases. Cell incubation with 4 mM hydroxyurea (Serva, United States) for 24 h caused cell cycle arrest at the G1/S-phase.
Cells at the S-phase were identified by labeling with 10 µM 5-bromo-2'-deoxyuridine (BrdU) (Serva, United States) for 24 h. The cells were fixed with 4% formaldehyde (MP Biochemicals, France) in 0.1 M PBS, pH 7.2 (Sigma, United States), treated with 4 N HCl, and stained with mouse monoclonal antibodies to BrdU (Digma, United States). The labeling index was determined by counting 3000 cells. p53 localization was identified with mouse monoclonal antibodies (Sigma, United States). Antibodies to mouse IgG conjugated with Alexa Fluor-488 (Sigma, United States) were applied as secondary antibodies. Nuclei were stained with 1µg/mL DAPI (Sigma, United States).
The specimens were analyzed using an Axiovert 200M fluorescent microscope (Carl Zeiss Inc., Images). Image processing was done using Adobe Photoshop and ImageJ.
RESULTS
Morphological description of entosis in MCF-7 and A431 cell lines. Entosis is a rather rare event in the population of breast adenocarcinoma MCF-7 and epidermoid carcinoma A431 cells. Light microscopic observations revealed various variants of entotic and engulfed cells in both cell cultures (Fig. 1, Table 1). Mono-, two-, and micronuclear entotic cells were encountered. Fourteen of 94 MCF-7 entotic cells had two nuclei. Fifteen of 71 A431 entotic cells were binuclear. The entotic vacuole could contain one or two internalized cells, as well as binuclear cells at various degradation steps. About 10% of entotic cells had two cells inside the vacuole (19 of 200 entotic events in the MCF-7 cell line and 27 of 200 entotic events in A431 cell line). About half of these cells were at stages 4–5 in the MCF-7 culture and exhibited evident degradation hallmarks (9 out of 19). In A431, culture only a quarter (7 of 27) of entotic cells manifested features of degradation.
Entosis in A431 cell culture. Light microscopy, hematoxylin and eosin staining. (a) Mononuclear entotic cell and invaded cell (stage 3), (b) binuclear entotic cell and degrading invaded cell (stage 4), (c) mononuclear entotic cell and binuclear invaded cell (stage 2), and (d) mononuclear entotic cell and two invaded cells with micronuclei (stage 2). E—entotic cell; I—invaded cell. Arrow points to an entotic vacuole. Scale bar is 10 µm.
Entotic mitotic cells were observed in both cultures (Figs. 2, 3, Table 1). Calculation of mitotic cells with the light microscopy showed that the mitotic index (MI) was higher in the A431 cell line than in MCF-7. In A431 cells, both entotic and invading cells entered into division more rarely than in the MCF-7 culture (Table 1).
Both cultures had cells at the late telophase that formed “craters” in interphase cells (Fig. 4). Crater formation is the initial step in engulfment (Garanina et al., 2017). In interphase cells, the bean-shaped nucleus was shifted to the periphery, which is a common hallmark of entosis (Figs. 4a–4c). Chromosome decondensation was observed at the late interphase stage (nucleoli were not indentified). The plasma membrane had numerous spherical protrusions. The cells remained connected by the residual body (Figs. 1a'–1c'). Sister cells may form a crater in one or two interphase cells, or a daughter cell may adhere to the substrate.
Initial stage of cell invasion at the late telophase in A431 culture. Light microscopy, hematoxylin and eosin staining. (a–c) Lower optical sections; (a'–c') upper optical sections of the same cells; a, a'–daughter cells form a crater in the interphase cells, their membranes produce numerous protrusions; (b, b')—one daughter cell forms a crater in the interphase cell; the other is attached to the substrate, arrow points to the residual body; and (b, b')—daughter cells form craters in neighbor interphase cells, arrow points to the residual body.
Entosis and invaded cell are capable of DNA replication. There are fragmentary data that thymidine is able to be incorporated into cell-in-cell structures (Brouwer et al., 1984). The entotic and invading cell’s capacity for cell cycle progress was analyzed by means of pulse incorporation of BrdU in cells at the S-phase.
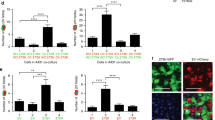
The labeling index was 30 ± 2.4 and 35 ± 5% in MCF-7 and A431 cells, respectively. BrdU incorporation demonstrated that both entotic and engulfed cells are capable of DNA synthesis (Figs. 5a–5d). Replication could take place simultaneously in both entotic and invading cells as well as only in entotic or only in invading cells.
BrdU incorporation in entotic and invaded MCF cells. (a) Entotic and invaded cells are at the S phase of the cell cycle, (b) entotic cell at the S phase, (c) lack of DNA replication in entotic and invaded cell, and (d) invaded cell at the S phase. Staining with antibodies to BrdU (green), nuclei are stained with DAPI (blue). White dotted contour outlines entotic cells and entotic vacuoles. E—entotic cell; I—invaded cell. (b) Scale bar is 10 µm. (e) Frequency of homo- and heterophase entoses in A431 (black columns) and MCF (grey columns) cultures. E—entotic cell without DNA replication, E+—entotic cell with DNA replication. I—invaded cell with DNA replication (n = 89 for А431; n = 69 for MCF7).
In MCF-7 culture (Fig. 5e), more than 50% of entoses were unlabeled; i.e., neither entotic nor engulfed cells replicated DNA. An amount of 29% entotic cells were in the S-phase of the cell cycle, which virtually coincided with the labeling index in the population (30 ± 2.4%). An amount of 26 of 40 entotic cells at stages 1–3 stayed in the S phase. Only 4 cells from 13 entosis cells at the stages 4—5 incorporated the label. Thus, in MCF-7 culture degradation of engulfed cells can affect entosis cell entry into S phase.
Entotic cells with replicated DNA were predominant (55%) in A431 culture unlike MCF-7 cells. It surpassed the labeling index in the population (35 ± 5%).
The labeling index of invading cells was lower than the population labeling index in both cultures (23 and 25% for MCF-7 and A431, respectively) (Fig. 5). Most invading cells with DNA replication were at stages 1–2. In MCF-7 culture, 12 of 19 invaded cells at stages 1–2 (about 63%) had incorporated BrdU label. No engulfed cells at later entosis stages (3–5) incorporated the label, probably due to active degradation processes.
Distribution of p53 protein in the entotic MCF-7 cell line. Cell cycle arrest is accompanied with p53 translocation to the nucleus. In MCF-7 culture, wild-type p53 protein was found in nuclei of 15.6 ± 0.1% cells (n = 1410). In entotic events, a variation of p53 distribution was observed: in nuclei of both entotic and invading cells, as well as only in nuclei of entotic cells or invading cells. Of 23 entotic mononuclear cells, positive nuclear staining was detected in six entotic (26%) and five engulfed (22%) cells. Entotic cells with micronuclei had p53 in the nucleus (Figs. 6d, 6d'). Thus, p53 was localized in the nucleus more frequently in entotic and invading cells than in the MCF-7 population at large.
Cell cycle disturbances do not prevent entosis. The cell cycle was arrested at the border of the G1 and S phases with hydroxyurea to assay whether the cells blocked at a cell cycle phase were capable of entosis. Hydroxyurea inhibits the activity of ribonucleotide reductase and DNA polymerases. Its treatment blocked the cell cycle at the border between G1 and S phases and inhibited S phase progression (Maurer-Schultze et al., 1988). The block was confirmed by the lack of BrdU incorporation into the cells. The block at the G1–S border did not affect the entosis frequency: 1.48 ± 0.5 (MCF-7) and 0.95 ± 0.3% (А431). This showed that entosis is not blocked by disturbances in the cell cycle progression.
Entosis and cell polyploidization, BrdU incorporation and the presence of mitoses indicated the cell cycle progression. Polyploid binuclear entotic and invading cells were found. The ploidy was assessed with the method of measurements of nuclear area projections. The projection area of the nuclei of interphase cells were assayed in both cultures (Figs. 7, 8) to estimate what cells could be considered polyploid. The results were compared with data from the Russian Cell Culture Collection of Vertebrates (RCCCV) for A431 cells. According to RCCCV (A431 cell source) 7% A431 cells are polyploid. Our measurements showed that 12.3 ± 2.4% cells in A431 culture had the nuclear size more than 200 µm2 (Fig. 8) that is consistent with RCCCV data. Nuclei in MCF-7 cell line had the larger projection area (only 2 cells had the nucleus size less 100 µm2). 26.6 ± 0.15% of MCF-7 cells had nuclei size more than 250 µm2 (Fig. 7). These findings coincide with RCCCV data and cells with the nucleus size larger 250 µm2 (MCF-7 cells) and more than 200 µm2 (A431 cells) could be referred to as polyploid cells. Cells with the least nuclear size (less than 150 µm2 for MCF-7 cells and 100 µm2 for A431 cells) could be presumably diploid cells in G1 phase of the cell cycle.
Distribution of nuclei for their projection area in MCF-7 cell culture. Horizontal—projection area of nuclei; vertical—cell number with certain projection area of nuclei, %. Black columns—nuclei distribution in interphase nonentotic cells (n = 800, vertical lines—standard deviation); grey columns—nuclei distribution in entotic cells (n = 93). Standard deviation was not determined as entosis was a rare event.
Distribution of nuclei for their projection area in A431 cell culture. Horizontal—projection area of nuclei; vertical—cell number with certain projection area of nuclei, %. Black columns—nuclei distribution in interphase nonentotic cells (n = 800, vertical lines—standard deviation); grey columns—nuclei distribution in entotic cells (n = 70). Standard deviation was not determined as entosis was a rare event.
Projection areas of entotic cell nuclei were also measured. In MCF-7 cells, the distribution of entotic cell nuclei was similar to the distribution of interphase nuclei of non-entotic cells. About 2.5% of entotic cells, like normal cells, were polyploid. Half of polyploid entotic cells (53.8%) had two nuclei. An increased number of mononuclear entotic cells that has nuclei of the smallest size (about 29%) were revealed (Fig. 7). These cells were probably in the G1 phase of the cell cycle.
The analysis of the nuclear projection areas in the A431 cell line showed that about 40% of cells were polyploid. The cells predominately had nuclei of the largest area (more than 300 µm2) (Fig. 8). Half of polyploid entotic cells (48.4%) were binuclear, as was observed in the MCF-7 cell line.
Thus, polyploid entotic cells were found in both cultures. We compared the projection area of entotic cells' nuclei with degradation of engulfed cells to elucidate whether polyploidy is associated with the entotic process. The degradation of invading cells took place for 24–48 h (Overholtzer et al., 2007; Garanina et al., 2017). This time is longer than the cell cycle length, and entotic cells may undergo mitotic division resulting in polyploidization. The level of degradation of invading cells was assessed on the basis of diminished projection of its nuclear area and increased size of the free vacuole space between entotic and engulfed cells (Fig. 9). On one hand, the longer the invaded cell stays inside the entotic cell, the more degraded it becomes. However, on the other other hand, the longer entosis lasts, the higher the probability that an entotic cell will enter mitosis and its nuclear size increase because of the chromosome segregation disorder. Thus, if polyploidization of entotic cells takes place during the entosis process, the nuclear size (polyploidization mark) of the entosis cells should be higher in the entotic cells with engulfed cells at the late stages of degradation (entosis duration mark).
Changes in nucleus size of invaded cells and entotic vacuole during the degradation. (a) Scheme of changes in nuclei size and entotic vacuoles during degradation. During degradation of an invaded cell, its nuclear size increases, as does the free space of the entotic vacuole. 1—entotic cell, 2—entotic cell nucleus, 3—invaded cell, 4—invaded cell nucleus, and 5—free space of entotic vacuole. (b) Nuclear size variation; entotic vacuole size variation, EV—entotic cell with nuclear size up to 200 (A431) or 250 µm2 (MCF-7), invaded cell does not exhibit signs of degradation; EV*—cell with nuclear size up to 200 (A431) or 250 µm2 (MCF-7), invaded cell exhibits degradation signs; polEV—polyploid entotic cell with nuclear size more than 200 (A431) or 250 µm2 (MCF-7), invaded cell does not exhibit signs of degradation; polEV*—polyploid entotic cell with nuclear size more than 200 (A431) or 250 µm2 (MCF-7), invaded cell exhibits signs of degradation. n = 95 for MCF-7 cells and 71 for A431 cells.
As mentioned above, entosis cells with nuclear size more than 250 (MCF-7 cells) and 200 µm2 (A431 cells) were considered polyploid. Engulfed cells with a nuclear projection area less than 80 µm2 in MCF-7 culture were considered degrading cells (the nuclear size of the interphase control cells was more than 80 µm2). In A431 culture, the cells with a nuclear size of less than 60 µm2 were considered as degrading (the nuclear size of control interphase cells was more than 64 µm2). In both cultures, engulfed cells with a size of the free vacuole space of less than 50 µm2 were considered degrading. .
In both cell lines, invading cells in nonpolyploid entotic cells are equally likely to have an unchanged structure of the nucleus or exhibit hallmarks of degradation (Fig. 9b). However, in polyploid entotic cells, the nucleus of the integrated cell is more frequently found at a stage of degradation. Analysis of entosis number with increased free vacuole space (Fig. 9c) showed that this process is common for both diploid and polyploid entotic cells. However, the percentage of vacuoles with free space is higher in polyploid than diploid entotic cells. Thus, in both cultures, engulfed cells in polyploid entotic cells more frequently underwent degradation. These findings may indicate an increase in the ploidy of entotic cells during entosis.
DISCUSSION
Entosis and cell cycle progression. Entosis has been reported for various tumor cells in vitro and in vivo. However, its biological significance remains largely elusive. Increased entosis number predicts poor prognosis. It is likely that an entotic cell’s entry into mitosis will result in its polyploidization due to disordered cytokinesis (Krajcovic et al., 2011; Krajcovic and Overholtzer, 2012). The cell cycle progress of entotic and invading cells may affect tumor cell polyploidization and genetic instability in the cell population. The presence of wild-type p53, a key regulator of the cell cycle (Chumakov, 2007; Kopnin et al., 2008; Mello and Attardi, 2018), should have influence an on the cell cycle progression during the entosis process.
Our study showed that, in both cell cultures, entotic and engulfed cells progress through the cell cycle. It follows from incorporation of the replicative label and the presence of mitotic figures during the entotic process. A large vacuole can disturb the cell cycle progression of entotic cells in p53+ cell lines. We found that MCF-7 cells at the late entotic stage are incapable of S-phase entry. In this culture, cell cycling is possible only at the initial stages (1–3) of entosis. The long-lasting process of lysome-mediated degradation prevented the entotic cell entrance into the S phase. An engulfed cell inside the entotic vacuole also disturbed the mitotic division of the invaded cell. The microtubule system is often disorganized in invading cells. Images presented in some publications (Krajcovic et al., 2011) show microtubules in engulfed cells, but these authors did not discuss these findings. It is most likely that the internalized cells entered into mitosis are not able to form the normal division spindle that, in turn, should disturb the chromosome segregation, resulting in polyploidy. The exit of such cells from the entotic vacuole after mitosis completion may increase the number of polyploidy cells in the population. Thus, both entotic and invading cells may be a source of genetic instability and stimulate tumor progression.
Polyploidization of entotic cells. Cell polyploidization is a symptom of cell cycle disorders. Our investigation revealed that both cultures had entotic cells. About half of these cells were binuclear cells presumably resulted from disturbed cytokinesis of entotic cells (Krajcovic et al., 2011). Half of polyploid cells were binuclear, and others with large nuclei, indicated that the entotic vacuole disturbed cytokinesis and chromosome segregation.
The number of entotic polyploid cells depended on the presence of wild-type p53. Unlike MCF-7 culture (p53+), A431 culture (p53–) had a significant percentage of entotic polyploid cells. Supposedly, polyploidization of A431 entotic cells resulting from their cycling, whereas MCF-7 cells were arrested in the G1 phase. The number of entotic cells in the S phase is higher in A431 than in MCF-7 culture. Engulfed degrading cells are an additional source of nutrients for entotic cells (Krajcovic et al., 2013; Durgan et al., 2017; Hamann et al., 2017;) that stimulate cell entry into replication. Unlike the A431 cell line, degradation of invaded cells in MCF-7 cells (p53+) activates p53 protein and prevents entry into the S phase. Nevertheless, both mitotic and polyploid entotic cells are encountered in MCF-7 culture. Cell invasion into the cells at the G1 phase may inhibit their cell cycle progression, as p53 mostly regulates the G1/S checkpoint. Cells that were invaded at other cell cycle phases continue cell cycle, enter mitosis and undergo polyploidization.
Engulfment and integration of cells at various cell cycle phases. The mechanisms of cell invasion into another cell and its subsequent degradation are now rather well described. However, what causes the invasion and what cells are targets for invasion remain practically unexplored. Cells at certain cell cycle phase may be preferred for cell invasion.
Invasion targets. There are fragmentary data on target cell characteristics. Entotic cells in pancreatic tumor had markers of epithelial cells (ЕМА, АЕ1Е3), fibroblasts (vimentin), and phagocytes (CD68) (Cano et al., 2012). However, these authors did not discuss the origin of entotic cells.
Supposedly, in cultures such as A431, the probability of becoming a target for invasion will be higher in cells with a larger area (polyploid cells). This explains the significant proportion of polyploid entotic cells.
In MCF-7 culture, an increased number of entotic cells with a very small size nucleus may indicate that the targets are cells at the G1 phase. The mechanism of this process is unclear. It can be suggested that these cells have enhanced expression of E-cadherin in the G1 phase, as the interaction with invading cells requires formation of adhesive contacts. The β-catenin-dependent cell cycle arrest in G1 phase (Stockinger et al., 2001) produced by E-cadherin hyperexpression is an indirect confirmation. MCF-7 cells arrested in G1 phase by p53 activation probably expressed more cadherin and were suitable targets for invasion. However, the opposite is also possible. Entotic cells with cells engulfed at the G1 phase hyperexpressed cadherins and form multiple contacts with invading cells, which results in its degradation. Degradation delay and entotic vacuole inhibit cell cycling. It can be suggested that entosis induces p53 activation, which follows from the increased number of p53+ cells.
Invading cells. It is virtually unknown why some cells in the population acquire a spherical shape and capacity for invasion. Supposedly, a critical factor is disorders in a cell’s contact with the extracellular matrix. A ball-like cell in contact with a spread cell retains the ability to produce adhesive contacts and desmosomes (Overholtzer et al., 2007; Garanina et al., 2017), which allows it to be introduced into a future entotic cell.
At certain stages of the cell life cycle, it loses polarity and is in contact with the matrix. The formation of daughter cells in the late telophase is accompanied with rearrangements of submembrane actin cytoskeleton. A significant amount of integrin receptors are absorbed by endocytosis (Pellinen et al., 2008; Paul et al., 2015). Daughter cells at this stage are ball-shaped with numerous membrane protrusions on the surface. The invasion of daughter cells ending mitosis was described for 16HBE and MCF-7 cultures (Durgan et al., 2017; Durgan and Florey, 2018). The cells forming a crater in interphase cells that we observed in the late telophase also may show the invasion process. However, additional studies with application of image correlation technique and scanning electron microscopy are needed to confirm cell invasion at this stage. Thus, the late telophase is a cell cycle phase that facilitates cell invasion.
There may be cell cycle phases that promote cell invasion, but further investigation is required to establish them.
In conclusion, an increased number of entotic cells with a very small nuclear size in MCF-7 culture may be explained by p53-mediated arrest in the cycle or predominant invasion in cells at G1 phase. In A431 (p53–) culture, an enhanced proportion of polyploid cells results from polyploidization during entosis under a disordered cell cycle, as well as predominant invasion into polyploid cells.
Entotic and engulfed cells are capable of cell cycle progression. The entosis process can lead to polyploidization of entotic and invaded cells. The presence of wild-type p53 protein partially inhibits polyploidization of the entotic cell and/or arrests the cell cycle in the G1 phase. In tumors with wild-type p53, entosis contributes less to polyploidization and affects the genetic instability than in p53-tumor cells.
REFERENCES
Brouwer, M., de Ley, L., Feltkamp, C.A., Elema, J., and Jongsma, A.P., Serum-dependent “cannibalism” and autodestruction in cultures of human small cell carcinoma of the lung, Cancer Res., 1984, vol. 44, pp. 2947–2951.
Cano, C.E., Sand, M.J., Hamidi, T., Calvo, E.L., Turrini, O., Bartholin, L., Loncle, C., Secq, V., Garcia, S., Lomberk, G., Kroemer, G., Urrutia, R., and Iovanna, J.L., Homotypic cell cannibalism, a cell-death process regulated by the nuclear protein 1, opposes to metastasis in pancreatic cancer, EMBO Mol. Med., 2012, vol. 4, pp. 964–979.
Chumakov, P.M., Protein p53 and its universal functions in a multicellular organisms, Usp. Biol. Khim., 2007, vol. 47, pp. 3–52.
Durgan, J. and Florey, O., Cancer cell cannibalism: Multiple triggers emerge for entosis, Biochim. Biophys. Acta, 2018, vol. 1865, pp. 831–841.
Durgan, J., Tseng, Y.Y., Hamann, J.C., Domart, M.C., Collinson, L., Hall, A., Overholtzer, M., and Florey, O., Mitosis can drive cell cannibalism through entosis, Elife, 2017, vol. 11. pii e27 134.
Fais, S., Cannibalism: a way to feed on metastatic tumors, Cancer Lett., 2007, vol. 258, pp. 155–164.
Garanina, A.S., Kisurina-Evgenieva, O.P., Erokhina, M.V., Smirnova, E.A., Factor, V.M., and Onishchenko, G.E., Consecutive entosis stages in human substrate-dependent cultured cells, Sci. Rep., 2017, vol. 7, p. 12 555.
Hamann, J.C., Surcel, A., Chen, R., Teragawa, C., Albeck, J.G., Robinson, D.N., and Overholtzer, M., Entosis is induced by glucose starvation, Cell Rep., 2017, vol. 20, pp. 201–210.
Kopnin, B.P., Kopnin, P.B., and Agapova, C.S., Multifaced p53: variety of forms, functions, tumor-suppressive and oncogenic activities, Klin. Onkogematol., 2008, vol. 1, pp. 2–9.
Krajcovic, M. and Overholtzer, M., Mechanisms of ploidy increase in human cancers: a new role for cell cannibalism, Cancer Res., 2012, vol. 72, pp. 1596–1601.
Krajcovic, M., Johnson, N.B., Sun, Q., Normand, G., Hoover, N., Yao, E., Richardson, A.L., King, R.W., Ci-bas, E.S., Schnitt, S.J., Brugge, J.S., and Overholtzer, M., A non-genetic route to aneuploidy in human cancers, Nat. Cell Biol., 2011, vol. 13, pp. 324–330.
Krajcovic, M., Krishna, S., Akkari, L., Joyce, J.A., and Overholtzer, M., mTOR regulates phagosome and entotic vacuole fission, Mol. Biol. Cell, 2013, vol. 24, pp. 3736–3745.
Maurer-Schultze, B., Siebert, M., and Bassukas, I.D., An in vivo study on the synchronizing effect of hydroxyurea, Exp. Cell Res., 1988, vol. 174, pp. 230–243.
Mello, S.S. and Attardi, L.D., Deciphering p53 signaling in tumor suppression, Curr. Opin. Cell Biol., 2018, vol. 51, pp. 65–72.
Overholtzer M. and Brugge, J.S., The cell biology of cell-in-cell structures, Nat. Rev. Mol. Cell Biol., 2008, vol. 9, pp. 796–809.
Overholtzer, M., Mailleux, A.A., Mouneimne, G., Normand, G., Schnitt, S.J., King, R.W., Cibas, E.S., and Brugge, J.S., A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion, Cell, 2007, vol. 131, pp. 966–979.
Paul, N.R., Jacquemet, G., and Caswell, P.T., Endocytic trafficking of integrins in cell migration, Curr Biol., 2015, vol. 25, pp. R1092–R1105.
Pellinen, T., Tuomi, S., Arjonen, A., Wolf, M., Edgren, H., Meyer, H., Grosse, R., Kitzing, T., Rantala, J.K., Kallioniemi, O., Fossler, R., Kallio, M., and Ivaska, J., Integrin trafficking regulated by Rab21 is necessary for cytokinesis, Dev. Cell, 2008, vol. 15, pp. 371–385.
Reiss, M., Brash, D.E., Munoz-Antonia, T., Simon, J.A., Ziegler, A., Vellucci, V.F., and Zhou, Z.L., Status of the p53 tumor suppressor gene in human squamous carcinoma cell lines, Oncol. Res., 1992, vol. 4, pp. 349–357.
Sharma, N. and Dey, P., Cell cannibalism and cancer, Diagn. Cytopathol., 2011, vol. 39, pp. 229–233.
Stockinger, A., Eger, A., Wolf, J., Beug, H., and Foisner, R., E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity, J. Cell Biol., 2001, vol. 154, pp. 1185–1196.
Yang, Y.-Q. and Li, J.-C., Progress of research in cell-in-cell phenomena, Anat. Rec., 2012, vol. 295, pp. 372–377.
ACKNOWLEDGMENTS
This study was supported by the Russian Science Foundation, project no 14-50-00029.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by I. Fridlyanskaya
Rights and permissions
About this article
Cite this article
Kisurina-Evgenieva, O.P., Khashba, L.A., Mamichev, I.A. et al. Entosis and Cell Cycle in Tumor Cell Culture. Cell Tiss. Biol. 13, 8–17 (2019). https://doi.org/10.1134/S1990519X19010073
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X19010073