Abstract
Under controlled conditions, the effect of high (40°C, 2 h) and positive low (4°C, 2 h) temperatures on the ultrastructure of mesophyll cells of the leaf and the content of photosynthetic pigments, phenols, and flavonoids in 2-week-old Triticum spelta plants was studied. The ultrastructure of the mesophyll cells of the leaf of the control plants was typical: a developed thylakoid system was clearly seen that was immersed in a fine-grained stroma in the chloroplasts of regular lenticular shape. Short-term hyperthermia caused a partial destruction of thylakoid membranes. Wave-shaped packing of grana thylakoids, significant expansion of lumen intervals, disturbance of the structural bond between the grana thylacoids and stroma thylakoids was noted. With hyperthermia, the mitochondria noticeably “swelled,” while the cristae membranes became less contrasting. The number of lipid droplets increased in the cytoplasm of cells. In the leaves, the content of chlorophylls and carotenoids decreased, however, the number of common phenols and flavonoids increased. Short-term hypothermia caused intense formation of plastoglobules, and an increase in the number and size of starch grains. Destruction of thylakoid membranes was not observed. Some of the mitochondria were rounded (40%), with their size being close to the control values, and some organelles were lenticular, “dumbbell,” and “cup-shaped.” Under hyper- and hypothermia, the T. spelta leaf mesophyll cells showed a tendency to increase the degree of chromatin condensation in the nucleus. Under hypothermia, the content and ratio of chlorophylls and carotenoids in leaves did not differ much from the control plants, and no significant quantitative changes in the total phenols and flavonoids were recorded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Under natural conditions, plant organisms are exposed to unfavorable environmental factors, which substantially limit their vital activity. These factors change physiological processes and activate the systems of adaptation to unfavorable conditions (Venzhik et al., 2012). Extreme temperatures are one of the most common abiotic stressors that cause morphological, physiological, and molecular changes and affect the growth and productivity of plants (Hatfield and Prueger, 2015). The photosynthetic apparatus is the most sensitive to the temperature regime (Kislyuk et al., 2007, 2008). Changes in the ultrastructure of mesophyll cells depend on the intensity and duration of temperature stress action, plant species, and its stress-resistance (Kislyuk et al., 2007; Salem-Fnayou et al., 2011; Popov et al., 2016). It has been noted that the effects of local temperature may be manifested in organs and parts of plants that are not directly exposed to stress (Veselova et al., 2003; Venzhik et al., 2017).
Chloroplasts are the most important sources of signals for other organelles and whole cells. For them, signaling is associated, first of all, with the photosynthetic function, and, since the intensity of photosynthesis is influenced by various factors, signals from chloroplasts serve as environmental sensors (Kleine et al., 2009). In the mesophyll cells of wheat leaves, hyperthermia causes changes in the morphology of the chloroplasts, influencing the size of the thylakoids and the granularity of the organelles (Kislyuk et al., 2007, 2008; Salem-Fnayou et al., 2011; Babenko et al., 2018), leading to the accumulation of plastoglobules and lipid droplets in the cytoplasm (Kislyuk et al., 2008). In plants of various species, a decrease in the amount of starch in chloroplasts was also noted (Salem-Fnayou et al., 2011; Klimchuk et al., 2012). High temperature has an effect on other cellular organelles. Thus, there have been seen a decrease in the number of cristae in the mitochondria of Oryza sativa leaf cells (Pareek et al., 1997) and a decrease in the electron density of the mitochondrial matrix of the Zea mays root cells and Valerianella locusta root cells (Ciamporova and Mistrik, 1993). In the endosperm of Z. mays, changes in the morphology of the nucleus, the electron density of the nucleoplasm, and the appearance of nucleoli without a granular component were noted (Commuri and Jones, 1999).
Under low temperature, the formation of large chloroplasts with a thylakoid system of “light type” occurred in mesophyll cells of wheat leaf (Venzhik et al., 2012). In wheat and other plants, the size and quantity of starch grana increased (Klimchuk et al., 2011; Babenko et al., 2018). It was reported that the chloroplast granularity was violated in tobacco (Popov et al., 2016) and the integrity of chloroplast membranes in cold-sensitive maize (Sopher et al., 1999) and tomatoes, beans, and tobacco plants (Holaday et al., 1992; Brüggemann et al., 1994; Popov et al., 2016). At the same time, the cold-resistant Pisum sativum and Brassica oleracea plants (Wise and Naylor 1987), as well as winter rye and rape plants (Hurry et al., 1995), showed swelling of plastids without damage to their membrane integrity. Among the reactions of other organelles to negative temperature, mitochondrial swelling was observed without compromising the integrity of membranes with the disappearance of the crista system in Brassica napus (Stefanowska et al., 2002) and Arabidopsis thaliana (Ristic and Ashworth, 1993). At a temperature of 5°C in A. thaliana, other authors observed a decrease in the volume of mitochondria (Armstrong et al., 2006). Cold-resistant plants have a change in the shape of the mitochondria (Vella et al., 2012; Babenko et al., 2018). Such diverse reactions of cellular organelles to temperature stresses mean the formation of different survival strategies under unfavorable temperatures (Kosakivska et al., 2008).
The success of adaptation to stressors in plants depends to a large extent on the functioning of the assimilation apparatus, indicators of which are the content and ratio of photosynthetic pigments (Babenko et al., 2014). It was reported that the level of chlorophylls in the leaves of wheat plant decreased during the first hour of cold exposure and gradually recovered in the next 24 h (Venzhik et al., 2012). A correlation between the thermal stability of winter wheat and the ratio of chlorophylls (a/b) and chlorophylls (a + b)/carotenoids was found (Babenko et al., 2014; Kosakovskaya et al., 2014).
In addition to changes in pigment content, thermal stress disrupts homeostasis in cells, which in turn leads to secondary oxidative stress and formation of reactive oxygen species (Asada, 2006). All phenolic compounds to some extent participate in the antioxidant defense of cells. They are accumulated mainly in vacuoles, chloroplasts, and the nucleus. According to the generally accepted view, the antioxidant features of phenolic compounds are explained by the unique structure of their molecules, which are capable of binding free radicals (Es-Safi et al., 2007). It was shown that the active accumulation of phenolic compounds directly depends on the functional activity and ultrastructural organization of chloroplasts. It is these cell organelles that regulate the biogenesis of flavonoids, which are the phenols that are most commonly present in the aerial parts of higher plants. Among all secondary metabolites of phenolic nature, flavonoids have the greatest antioxidant and radical neutralizing potential, they protect cells from reactive oxygen species, prevent lipid peroxidation, protein denaturation, and DNA damage (Król et al., 2015). However, at present the question of the role of phenolic compounds in protecting plants from unfavorable temperatures remains open.
Wheat is the world’s second-largest crop. In today’s wheat production, a tendency has emerged to revive, select, and introduce into production forgotten regional cereals, so-called “ancient cereals” (Babenko et. al., 2018), such as T. spelta. Due to its valuable food and economic features, this culture is experiencing a second birth.
Earlier, we investigated the effects of hyper- and hypothermia on the ultrastructural organization and spectrum of photosynthetic pigments in the new highly productive Triticum aestivum varieties (Babenko et al., 2014, 2018). In the present work, we studied the nature of changes in the ultrastructure of leaf mesophyll cells, the content of photosynthetic pigments, and secondary metabolites, which are phenols and flavonoids in T. spelta in the initial period of stress temperatures action to elucidate the possible role of structural and functional features in the formation of an adaptive response in wild congener of T. aestivum.
MATERIALS AND METHODS
Plant material and growing conditions. Experiments were carried out with 14-day-old T. spelta (2n = 42) plants, var. Frankenkorn, created in the 1990s on the basis of old varieties of spell by reverse crossing. The variety is medium-sized, resistant to lodging and excessive moisture, frost-resistant, ecologically plastic, and genetically pure. Seeds were obtained from the collection of the National Center for Plant Genetic Resources of Ukraine (Kharkyv). The seeds were washed in distilled water, transferred to Petri dishes on a filter paper moistened with Knop’s solution, and placed in a thermostat at 24°C in the dark. A day later, the germinated seeds were transferred to a climate chamber, where they grew for 14 days at 25°C, relative humidity of 60–70%, illumination of 180 μmol/(m2 s), and a photoperiod of 16/8 h (day/night). To create heat and cold stresses, 14-day-old plants were exposed to short-term (2 h) exposure to temperatures of 40 and 4°C, respectively, under the indicated regime of humidity and illumination.
For electron microscopic studies, cuttings 1 × 2 mm in size were used from the middle part of the second leaf. The samples were preliminarily fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) under vacuum infiltration at room temperature (for 1 h) and then at 4°C for 4 h. The samples were washed in the same buffer and postfixed with a 1% solution of OsO4 in 0.1 M cacodylate buffer (pH 7.2) at 4°C for 12 h. For dehydration, solutions of ethyl alcohol of increasing concentration were used, and, after treatment with acetone, they were enclosed in a mixture of epoxy resins of Epon and Araldite. Sections obtained on an LKB-8800 ultramicrotome (Sweden) were analyzed on a JEM-1230 electron microscope (JEOL, Japan) at an accelerating voltage of 80 kV. For the morphometric analysis of cells and organelles, UTHSCSA Image Tool 3 (USA) with a scale line of electron microscopic images was used. In each variant, at least 100 electron microscopic images were analyzed.
The photosynthetic pigments were extracted with 80% acetone and determined by the Wellburn method (1994).
The phenol content was determined using a Folin-Ciocalteu reagent (Bobo-García et al., 2015). Gallic acid was used as the standard for constructing the calibration curve. The content of flavonoids was determined using a method based on the reaction of flavonoids with zirconyl (IV) nitrate hydrate (Smirnov et al., 2015).
Statistical analysis of the results was carried out using One-way ANOVA. Differences were considered significant at P ≤ 0.05, 0.01, and P ≤ 0.001. The values presented correspond to the mean and their standard errors.
RESULTS AND DISCUSSION
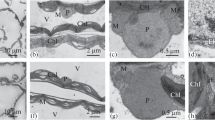
The mesophyll cells of the leaves of the 14-day-old T. spelta plant, var. Frankenkorn, had an elongated-oval shape. The cytoplasm of cells was represented by a narrow layer located along the cell wall with organelles immersed in it and a large central vacuole. Chloroplasts were located along the plasma membrane. On the diametrical sections of mesophyll cells of control plants and treated with short-term temperature stresses, 10–11 chloroplasts were found on average (Table 1). Chloroplasts of control plants were oval in shape. Their grana consisted of closely packed thylakoids; stroma thylakoids extended from their terminal parts. Numerically, grana with 11–15 thylakoids predominated (Figs. 1a, 1b). Plastoglobules were found in chloroplasts, which were located near the stromal thylakoids, as well as a small amount of starch grains with a size of 0.15 ± 0.02 (Table 1). Lipid drops were present in the cytoplasm (Table 1). In the nucleus, electron-dense areas of condensed chromatin were identified (Fig. 1c).
Features of ultrastructure of mesophyll cells in Triticum spelta leaf. (a–c) Control, (d–f) hypothermia (4°С, 2 h), and (g–i) hyperthermia (40°С, 2 h). Chl—chloroplast, N—nucleus, M—mitochondria, LD—lipid drop, SG—starch grain, GT—grana thylakoids, ST—stroma thylakoids, G—grana, and Pg—plastoglobula.
After cold stress, the size of the mesophyll cells had increased somewhat (Table 1) and the chloroplasts acquired a more rounded shape (Fig. 1d). There were no significant changes in the thylakoid system. A thickening of the grana of the thylakoids and an increase in the width of the luminal space were noted (Table 2). However, the thylakoid grana were well developed and closely adhered to each other. A uniform arrangement of the grana in the stroma of the chloroplast was observed (Fig. 1e). After hypothermia, an increase in the number and size of starch grains in chloroplasts was observed (Table 1; Figs. 1d, 1e).
Other authors reported that the effect of low temperatures, as well as drought, caused the accumulation of sugars in plants (Yamada and Osakabe, 2017). The revealed changes may be connected with the damage to the system of outflow of assimilates (sucrose) under cold.
Short-term action of low temperature led to the appearance of numerous plastoglobules in the stroma of chloroplasts (Table 1; Fig. 1e). Their number was much higher than the corresponding values after the action of high temperature and in control. The increase in the number of plastoglobules in chloroplasts was related to nonspecific reactions, since it manifests itself under the action of various stress factors (Salem-Fnayou et al., 2011). Proteomic and ultrastructural studies indicated the participation of plastoglobules in the stabilization of thylakoid membranes under oxidative damage. They are a storage site for lipid-like substances (such as carotenoids, tocopherol, and plastoquinone) and specific proteins with enzyme and structural functions (Austin et al., 2006).
After thermal stress, the chloroplasts acquired a lenticular shape, the grana were unevenly distributed in the stroma, and partial destruction of the thylakoid membranes was observed, which was expressed in undulating packing of the grana thylakoids and a significant expansion of the luminal spaces. Disturbances in the structural connection between the stroma thylakoids and the grana thylakoids have been revealed (Table 2; Figs. 1g, 1h).
The number and volume of starch grains somewhat decreased (Table 1). In the cytoplasm, compared to control and hypothermia, the amount of lipid droplets increased. Similar effects were observed earlier in the mesophyll cells of the leaves of Brassica ampestris, Amaranthus caudatus, and T. aestivum, var. Volodarka (Kosakivska et al., 2008; Klimchuk et al., 2012, Babenko et al., 2014). The formation of numerous lipid droplets in mesophyll cells of T. spelta leaves under hyperthermia occurred against the increased activity of two membrane-associated isoforms of lipoxygenase, which is the key enzyme for the metabolism of polyunsaturated fatty acids (Babenko, 2018). Other authors also reported that high temperature and drought caused accumulation of lipid droplets and a decrease in the amount of starch in wheat chloroplasts (Vassileva et al., 2011). Under hyper- and hypothermia, the T. spelta leaf mesophyll cells showed a tendency to an increasing degree of chromatin condensation in the nucleus (Figs. 1f, 1i).
The mitochondria in the mesophyll cells of the control plants were rounded and characterized by an electron-dense matrix and numerous developed lamellar crystals (Fig. 2a). With the action of high temperature, on the contrary, the mitochondria noticeably “swelled” and the cristae membranes became less contrasting. A partial bleaching of the matrix of these organelles was observed (Fig. 2b). Their number increased (Table 1). After a brief hypothermia, there was obtained a significant change in the morphology of the organelles: some of the mitochondria (40%) retained a circular shape (Fig. 2c) and sizes close to the control ones; however, some organelles acquired a “cuplike” (Fig. 2d) or lenticular form (Fig. 2e), and mitochondria of the “dumbbell” shape also existed (Fig. 2f).
The mitochondrial form is a highly dynamic structural index (Logan, 2010). In thermophilic plants with cold stress, the change in the shape of the organelles was accompanied by a decrease in the number of cristae, which is regarded as a sign of their being damaged. In cold-resistant plants, such as Arabidopsis or soft wheat species, a change in the shape of the mitochondria to an elongated “dumbbell” and “cupped” shape is reversible (Vella et al., 2012; Venzhik et al., 2012). Under hypothermia, in the frost-resistant T. aestivum plant, var. Volodarka, we also recorded the formation of “dumbbell” mitochondria (Babenko et al., 2018). It has been suggested that this form of organelles promotes an increase in their surface area and facilitates the exchange of metabolites between organelles and cytoplasm (Vella et al., 2012).
It was shown that an increase in the size of mitochondria with short-term stress indicates an activating in the respiratory capacity of the cell (Armstrong et al., 2006; Venzhik et al., 2012). Other authors showed no increase in mitochondrial size during prolonged cooling of plants, but an increase in their number was noted (Venzhik et al., 2017).
In recent decades, ideas have been formed, according to which plant damage due to the temperature stress action begins with disturbances of the structure and functions of membranes. Membrane changes are the earliest reactions to hypothermia. It has been suggested that the key role in the formation of resistance to hypothermia is associated with an increase in the proportion of unsaturated fatty acids in the membrane lipids (Theocharis et al., 2012). Changes in the fatty acid composition of lipids are aimed at maintaining the fluidity of membranes at a level sufficient for the functioning of the photosynthetic and energy apparatus of the cell, which allows plants to survive under short-term extreme temperatures (Rurek, 2014). For example, the greater flexibility and elasticity of membranes of the frost-resistant plants, which apparently contain significant amounts of unsaturated fatty acids and allow mitochondria to actively change their volume, which provides the cell with a higher energy potential. Conversely, the lower flexibility of membranes, which are sensitive to cooling, prevents the cell from changing the rate of oxidation, contributes to a decrease in permeability for oxidation substrates, and leads to the accumulation of cell-damaging intermediates.
Photosynthesis is sensitive to any temperature changes, since temperature stress may be accompanied by an imbalance between the light energy absorbed by the photosystem and the energy consumed by metabolic processes. At optimum temperature, the efficiency of photosynthesis reaches 90% of its maximum value. For the same plant species, the temperature optimum of photosynthesis is unstable. It depends on the plant age, adaptation to certain temperatures and may vary during the season. The maximum temperature of photosynthesis is on average 10–15°С below the point of thermal depression. Thus, for most C3 plants in the temperate zone, the optimum temperature is in the range of 20–25°C (Voskresenskaya et al., 2014). The pigment complex reacts actively to signals of the surroundings, and changes in the content and proportion of pigments serve as a test that allows one to assess the effect of temperature on the plant state.
After short-term hypothermia in leaves of 2-week-old T. spelta plants, the content and ratio of chlorophylls and carotenoids was practically the same as the control one (Table 3). However, after short-term hyperthermia, the amount of chlorophylls and carotenoids decreased and the ratio of chlorophylls a/b increased by 12% (Table 3). The obtained data testify to the sensitivity of the plant pigment complex to the action of high temperature. In other cases, the total content of chlorophylls and the proportion of chlorophyll in the light complex in the leaves of wheat plant after 1 h of reduced temperature, but, after 24 h of cooling, gradual recovery of the level of green pigments was noted (Venzhik et al., 2012).
Earlier, it was shown that, in 2-week-old T. aestivum plants of the heat-resistant varieties, the total chlorophyll content decreased after cold stress (Kosakovskaya et al., 2014), while a decrease in the total content of chlorophylls after heat stress appeared in cold-resistant varieties (Babenko at al., 2014).
Short-term hypothermia did not cause any significant changes in the content of common phenols and flavonoids in the leaves of 14-day-old T. spelta plants. After short-term hyperthermia, an increase in the content of these compounds was observed (Fig. 3). An increase in the phenol content under hyperthermia appears to be due to imbalances between the formation of reactive oxygen species and the efficiency of antioxidant protection (Reddy et al., 2004).
According to the literature, the effects of temperature on the content of phenols are contradictory. Thus, after heat stress (35°C), in Lycopersicon esculentum plants, for which the optimal growth temperature is 22–26°C, and after cold stress in Citrullus lanatus plants with an optimum growth temperature of 33–35°C, phenols were accumulated (Rivero et al., 2001). It was reported that leaves of a cold-sensitive grape variety were characterized by a lower content of total phenols than leaves of a sustainable variety (Król et al., 2015). Under cold stress, a higher level of phenols was found in the tissues of the thermophilic Rehmannia glutinosa plant and grapes (Weidner et al., 2009; Amarowicz et al., 2010). In some publications, it was reported that abiotic stress induced increase in the synthesis of phenolic compounds in plant tissues (Swigonska et al., 2014). In other works, on the contrary, it was shown that cold stress either did not cause significant changes in the content of phenolic compounds in pea roots (Rudikovskaya et al., 2008) or led to a decrease in this characteristic (Posmyk et al., 2005). At low-temperature adaptation of wheat plants, a significant increase in the content of flavonoids was noted, especially in the frost-resistant variety (Olenichenko et al., 2008). At the same time, under low damaging temperatures, a decrease in the content of flavonoids was recorded in potato plants (Pavlyuchkova et al., 2014). A correlation was reported between the resistance of cultivar varieties and the content of flavonoids (Treutter, 2006). It was shown that, in the cold-resistant wheat variety, the content of flavonoids after cold hardening was significantly higher than that of the unstable one. However, in nonhardened plants, such differences were not revealed (Olenichenko et al., 2008).
Comparison of ultrastructural rearrangements in mesophyll cells of T. spelta leaves under short-term hypo- and hyperthermia made it possible to conclude that they are the “trigger mechanism” of various adaptive programs. Comparison with our results, as well as literature data, suggested that some of the ultrastructural rearrangements occurring under low and high temperature that are common to cold-resistant ones (Vella et al., 2012; Venzhik et al., 2012) and heat-resistant species (Klimchuk et al., 2011, 2012; Popov et al., 2016; Babenko et al., 2018) are also characteristic for spell. These include a change in the morphology and ultrastructural organization of chloroplasts and mitochondria, an increase in the size and quantity of starch grains, and an increase in the number of plastoglobules and lipid droplets in the hyaloplasm. In particular, changes in the structural organization of chloroplasts are accompanied by an increase in the stroma density of the organelles, indicate rapid changes in its chemical composition (Li et al., 2011; Vella et al., 2012) and are an indirect indicator of the intensification of metabolic processes under stress.
One indicator of plant resistance to stress is the content of phenols (Król et al., 2015), the pool of which depends on the duration of stress, its intensity, the phase of plant development, and what object underwent to stressor effects: the whole plant or its separate organs (Weidner et al., 2009). The increase in the phenol content under hyperthermia in our studies may be a protective reaction associated with imbalance between the formation of reactive oxygen species and the efficiency of antioxidant protection (Reddy et al., 2004).
Thus, our results show that the reaction of the mesophyll cells of the T. spelta leaf to the action of extreme temperatures is expressed in the structural and functional reorganization of the photosynthetic and energy apparatus, which is started in the first hours of stress temperature action on the plant and, it seems to us, is a necessary condition for the implementation of adaptive programs.
REFERENCES
Amarowicz, R., Weidner, S., Wojtowicz, I., Karmacґ, M., Kosinґska, A., and Rybarczyk, A., Influence of low-temperature stress on changes in the composition of grapevine leaf phenolic compounds and their antioxidant properties, Funct. Plant Sci. Biotechnol., 2010, vol. 4, pp. 90–96.
Armstrong, A.F., Logan, D.C., Tobin, A.K., O’Toole, P., and Atkin, O.K., Heterogeneity of plant mitochondrial responses underpinning respiratory acclimation to the cold in Arabidopsis thaliana Leaves, Plant Cell Environ., 2006, vol. 29, pp. 940–949.
Asada, K., Production and scavenging of reactive oxygen species in chloroplasts and their functions, Plant Physiol., 2006, vol. 141, pp. 391–396.
Austin, J.R., Frost, E., Vidi, P.A, Kessler, F., and Staehelin, L.A., Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes, Plant Cell, 2006, vol. 18, pp. 1693–1703.
Babenko, L.M., The effect of stress temperatures on the activity of lipoxygenases in Triticum spelta, Bull. Kharkiv Nat. Agrar. Univ., Ser. Biol., 2018, vol. 1, no. 43, pp. 40–46.
Babenko, L.M., Kosakivska, I.V., Akimov, Yu.A., Klymchuk, D.O., and Skaternya, T.D., Effect of temperature stresses on pigment content, lipoxygenase activity and cell ultrastructure of winter wheat seedlings, Gen. Plant Physiol., 2014, vol. 4, pp. 117–125.
Babenko, L.M., Scherbatiuk, N.N., Klimchuk, D.A, and Kosakovskaya, I.V., Structural-functional peculiarities of leaf mesophyll cells of Triticum aestivum cultivars with different cold/heat tolerance under short-term temperature stresses, Tsitologiia, 2018, vol. 60, no. 2, pp. 128–135.
Bobo-García, G., Davidov-Pardo, G., Arroqui, C., Vírseda, P., Marín-Arroyo, M.R., and Navarro, M., Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods, J. Sci. Food Agric., 2015, vol. 95, pp. 204–209.
Brüggemann, W., Klaucke, S., and Maas-Kantel, K., Long-term chilling of young tomato plants under low light. V. Kinetic and molecular properties of two key enzymes of the Calvin cycle in Lycopersicon esculentum Mill. and L. peruvianum Mill, Planta, 1994, vol. 194, pp. 160–168.
Ciamporova, M., and Mistrik, I., The ultrastructural response of root cells to stressful conditions, Environ. Exp. Bot., 1993, vol. 33, pp. 11–26.
Commuri, P.D. and Jones, R.J., Ultrastructural characterization of maize (Zea mays L.) kernels exposed to high temperature during endosperm cell division, Plant Cell Environ., 1999, vol. 22, pp. 375–385.
Es-Safi, N.E., Ghidouche, S., and Ducrot, P.H., Flavonoids: hemisynthesis, reactivity, characterization and free radical scavenging activity, Molecules, 2007, vol. 12, pp. 2228–2258.
Hatfield, J. and Prueger, J., Temperature extremes: effect on plant growth and development, Weather Climate Extremes, 2015, vol. 10, pp. 4–10.
Holaday, A.S., Martindale, W., and Alred, R., Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature, Plant Physiol., 1992, vol. 98, pp. 1105–1114.
Hurry, V.M., Strand, Å., and Tobiæson, M., Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content, Plant Physiol., 1995, vol. 109, pp. 697–706.
Kislyuk, I.M., Bubolo, L.S., Kamentseva I.E., Kotlova, E.R., and Sherstneva, O.A., Heat shock increases thermotolerance of photosynthetic electron transport and the content of chloroplast membranes and lipids in wheat leaves, Russ. J. Plant Physiol., 2007, vol. 54, pp. 456–463.
Kislyuk, I.M., Bubolo, L.S., Bykov, O.D., Kamentse-va, I.E., and Sherstneva, O.A., Protective and injuring action of visible light on photosynthetic apparatus in wheat plants during hyperthermia treatment, Russ. J. Plant Physiol., 2008, vol. 55, pp. 613–621.
Kleine, T., Voigt, C., and Leister, D., Plastid signalling to the nucleus: messengers still lost in the mists?, Trends Genet., 2009, vol. 25, pp. 185–192.
Klymchuk, D.O., Kosakivska, I.V., Akimov, Yu.M., Shcherbatyuk, M.M., and Vorobyova, T.V., Structure-functional peculiarities of Brassica campestris and Amarantus caudathus leaf cells under low positive temperature, Bull. Kharkiv Nat. Agrar. Univ., Ser. Biol., 2011, vol. 3, no. 24, pp. 15–24.
Klymchuk, D.O., Kosakivska, I.V., Akimov, Yu.M., Shcherbatyuk, M.M., and Vorobyova, T.V., Structural and functional peculiarities of Brassica campestris and Amaranthus caudatus leaf cells under high temperature, Bull. Kharkiv Nat. Agrar. Univ., Ser. Biol., 2012, vol. 2, no. 26, pp. 61–70.
Kosakivska, I.V., Klymchuk, D.O., Negretzky, V.A., Bluma, D.A., and Ustinova, A.Yu., Stress proteins and ultrastructural characteristics of leaf cells in plants with different types of ecological strategies, Gen. Appl. Plant Physiol., 2008, vol. 34, pp. 405–418.
Kosakovskaya, I.V., Babenko, L.M, Skaternaya, T.D., and Ustinova, A.Yu., Influence of hypo- and hyperthermia on the activity and content of pigments and soluble proteins in Triticum aestivum L. seedlings of Yatran 60, Fiziol. Rast. Genet., 2014, vol. 46, no. 3, pp. 212–220.
Krol, A., Amarowiczb, R., and Weidnera, S., The effects of cold stress on the phenolic compounds and antioxidant capacity of grapevine (Vitis vinifera L.) leaves, J. Plant Physiol., 2015, vol. 189, pp. 97–104.
Li, T.A., Xu, S.L., Oses, Prieto, J.A., Putil, S., Xu, P., Wang, R.L., Li, K.H., Maltby, D.A., An, L.H., Burlingame, A.L., Deng, Z.P., and Wang, Z.Y., Proteomics analysis reveals posttranslational mechanisms for cold-induced metabolic changes in Arabidopsis, Mol. Plant., 2011, vol. 4, pp. 361–374.
Logan, D.C., Mitochondrial fusion, division and positioning in plants, Biochem. Soc. Trands., 2010, vol. 38, pp. 789–779.
Olenichenko, N.A., Zagoskina, N.V., Astakhova, N.V., Trunova, T.I., and Kuznetsov, Yu.V., Primary and secondary metabolism of winter wheat during cold hardening and the action of antioxidants, Appl. Biochem. Microbiol., 2008, vol. 44, no. 5, pp. 589–594.
Pareek, A., Singla, S., and Grover, A., Short-term salinity and high temperature stress associated ultrastructural alterations in young leaf cells of Oryza sativa L., Ann. Bot., 1997, vol. 80, pp. 629–639.
Pavlyuchkova, S.M., Spivak, E.A., Vershilovskaya, I.V., Nedved, E.L., and Shkraba, E.V., Effect of exogenous 5-aminolevulinic acid on the functioning of the antioxidant system of potato plants (Solanum tuberosum) under low-temperature stress, Vestsi Nat. Acad. Nauk. Belarusi, Ser. Biyal., 2014, vol. 3, pp. 46–51.
Popov, V.N., Antipina, O.V., and Astakhova, N.V., Changes in chloroplast ultrastructure of tobacco plants in the course of protection from oxidative stress under hypothermia, Russ. J. Plant Physiol., 2016, vol. 63, pp. 301–307.
Posmyk, M.M., Bailly, C., Szafranґska, K., Jasan, K.M., and Corbinea, F., Antioxidant enzymes and isoflavonoids in chilled soybean (Glycine max (L.) Merr.) seedlings, J. Plant Physiol., 2005, vol. 162, pp. 403–412.
Reddy, A.R., Chaitanya, K.V., and Vivekanandan, M., Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants, J. Plant Physiol., 2004, vol. 161, pp. 1189–1202.
Ristic, Z. and Ashworth, E., Changes in leaf ultrastructure and carbohydrates in Arabidopsis thaliana L. (Heynh) cv. Columbia during rapid cold acclimation, Protoplasma, 1993, vol. 172, pp. 111–123.
Rivero, R., Ruiz, J., Garcıa, P., Lopez-Lefebre, L., Sanchez, E., and Romero, L., Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants, Plant Sci., 2001, vol. 160, pp. 315–321.
Rudikovskaya, E.G., Fedorova, G.A., Dudareva, L.V., Makarova, L.E., and Rudokovskij, A.V., Effect of growth temperature on the composition of phenols in pea roots, Russ. J. Plant Physiol., 2008, vol. 55, pp. 712–715.
Rurek, M., Plant mitochondria under a variety of temperature stress conditions, Mitochondrion, 2014, vol. 19, pp. 289–294.
Salem-Fnayou, A.B., Bouamama, B., Ghorbel, A., and Mliki, A., Investigations on the leaf anatomy and ultrastructure of grapevine (Vitis vinifera) under heat stress, Microsc. Res. Tech., 2011, vol. 74, pp. 756–762.
Smirnov, O., Kosyan, A., Kosyk, O., and Taran, N., Response of phenolic metabolism induced by aluminium (Al3+) toxicity in Fagopyrum esculentum Moench plants, Ukr. Biochem. J., 2015, vol. 87, pp. 129–135.
Sopher, C.R., Krol, M., and Huner, N.P.A., Chloroplastic changes associated with paclobutrazol-induced stress protection in maize seedlings, Can. J. Bot., 1999, vol. 77, pp. 279–290.
Stefanowska, M., Kura, M., and Kacperska, A., Low temperature induced modifications in cell ultrastructure and localization of phenolics in winter oilseed rape (Brassica napus L, var. oleifera) leaves, Annu. Bot., 2002, vol. 90, pp. 637–645.
Swigonska, S., Amarowicz, R., Kryl, A., Mostek, A., Badowiec, A., and Weidner, S., Influence of abiotic stress during soybean germination followed by recovery on the phenolic compounds of radicles and their antioxidant capacity, Acta Soc. Bot. Pol., 2014, vol. 83, pp. 209–218.
Theocharis, A., Clement, C., and Barka, E.A., Physiological and molecular changes in plants grown at low temperatures, Planta, 2012, vol. 235, pp. 1091–1105.
Treutter, D., Significance of flavonoids in plant resistance: a review, Environ. Chem. Lett., 2006, vol. 4, pp. 147–157.
Vassileva, V., Signarbieux, C., Anders, I., and Feller, U., Genotypic variation in drought stress response and subsequent recovery of wheat (Triticum aestivum L.), J. Plant Res., 2011, vol. 124, no. 1, pp. 147–154.
Vella, G.F., Joss, T, V., and Roberts, T.H., Chilling-induced ultrastructural changes to mesophyll cells of arabidopsis grown under short days are almost completely reversible by plant rewarming, Protoplasma, 2012, vol. 249, pp. 1137–1149.
Venzhik, Yu.V., Titov, A.F., Talanova, V.V., Mirosla-vov, E.A., and Koteeva, N.K., Structural and functional reorganization of the photosynthetic apparatus in adaptation to cold of wheat plants, Cell Tissue Biol., 2013, vol. 7, no. 2, pp. 168–176.
Venzhik, Yu.V., Titov, A.F., and Talanova, V.V., Short-term chilling of wheat seedlings or roots affects the ultrastructure of mesophyll cells, Tr. Karel. Nauch. Tsentra, Ross. Akad. Nauk, 2017, vol. 5, pp. 66–78.
Veselova, S., Farhutdinov, R., Mitrichenko, A., Symonyan, M., and Hartung, W., The effect of root cooling on hormone content and root hydraulic conductivity of durum wheat seedlings (Triticum durum L.), Bulg. J. Plant Physiol., Special Issue, 2003 pp. 360–366.
Voskresenskaya, O.L., Voskresenskiy, V.S., Sabaeva, E.V., and Yagdarova, O.A., Influence of ultraviolet radiation and microclimate parameters on the content of pigments in leaves of birch which grows under the conditions of the city, Vestn. Udmurt. Univ., Ser. Biol. Nauki Zemle, 2014, vol. 3, pp. 39–45.
Weidner, S., Kordala, E., Brosowska-Arendt, W., Karamacґ, M., Kosinґska, A., and Amarowicz, R., Phenolic compounds and properties of antioxidants in grapevine roots followed by recovery, Acta Soc. Bot. Pol., 2009, vol. 78, pp. 279–286.
Wellburn, A., The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution, J. Plant Physiol., 1994, vol. 144, pp. 307–313.
Wise, R.R. and Naylor, A.W., Chilling-enhanced photooxidation. The peroxidative destruction of lipids during chilling injury to photosynthesis and ultrastructure, Plant Physiol., 1987, vol. 8, pp. 272–277.
Yamada, K., and Osakabe, Y., Sugar compartmentation as an environmental stress adaptation strategy in plants, Semin. Cell Dev. Biol., 2017, vol. 72, pp. 1–10.
ACKNOWLEDGMENTS
This work was supported by the National Academy of Sciences of Ukraine, the project “The Phytohormonal System of New Genotypes of Triticum aestivum L. and Its Wild Ancestors under the Influence of Extreme Climatic Factors.”
We are thankful to D.A. Klimchuk, Ph.D., the head of the Center for Electron Microscopy, N. Kholodny Institute of Botany, National Academy of Sciences of Ukraine, for his attention and useful discussion of the results when this publication was prepared.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by T. Borisova
Rights and permissions
About this article
Cite this article
Babenko, L.M., Vodka, M.V., Akimov, Y.N. et al. Specific Features of the Ultrastructure and Biochemical Composition of Triticum spelta L. Leaf Mesophile Cells in the Initial Period of Stress Temperature Action. Cell Tiss. Biol. 13, 70–78 (2019). https://doi.org/10.1134/S1990519X19010024
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X19010024