Abstract
This study investigated the relationship between major depressive disorder (MDD) and neuroinflammation biomarkers. Included 28 patients that were diagnosed with MDD and 22 healthy individuals as the control group. Antidepressant drug therapy was started for depression patients. Venous blood was drawn from the control group and pre-treatment MDD patients for measuring levels of inflammatory markers. Inflammatory marker levels were measured again after 8 weeks of antidepressant medication in MDD patients. The severity of depression was determined by the Hamilton Depression Scale (HDS). Serum levels of IL-1, IL-2, IL-6 and haptoglobin were significantly different between the pre-treatment patients and control groups (P < 0.05), but no significant difference in CRP and sedimentation levels (P > 0.05). Pre- and post-treatment of patient group levels of IL-1, IL-2, IL-6 and haptoglobin significantly different (P < 0.05), whereas CRP levels and sedimentation were not significantly different (P > 0.05). Mean HDS score of pre- and posttreatment in patients group was significantly different (P < 0.05). All participants in the patient group responded to the antidepressant treatment. We found that inflammatory mechanisms occur in MDD and antidepressant therapy affects this process. Inflammatory cytokines IL-1, IL-2 and IL-6, as well as the APR’s haptoglobin are elevated significantly in MDD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Studies conducted in the field of psychoneuroimmunology over the last few decades have shown that there exists a two-way relation network between the immune system and the brain [1]. The immune system is composed of two subsystems, namely the innate immune system (inherited) and the adaptive immune system (acquired). Both subsystems involve cellular and humoral components, and these components secrete distinct cytokines. Interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, interferon (IFN)-α are examples of the cytokines produced by the innate immune system; whereas IL-2, IFN- γ, IL-4 and IL-10 are among the cytokines produced by the adaptive immune system. Cytokines can also be classified as those that augment the inflammatory response (proinflammatory cytokines) and those that alleviate the inflammatory response or counterpoise an elevated inflammatory response (anti-inflammatory cytokines). IL-1, IL-2, IL-6, TNF-α and IFN-γ are proinflammatory, while IL-4, IL-10 and IL-13 are anti-inflammatory cytokines. Furthermore, proinflammatory cytokines mediate secretion of acute-phase reactants (APRs), such as C-reactive protein (CRP), haptoglobin, complement component 3, fibrinogen and ceruloplasmin [2–5]. APRs are proteins serum concentrations of which either increase by at least 25% (positive APRs) or decrease (negative APRs) in response to inflammation. The examples of positive APRs are erythrocyte sedimentation rate (ESR), CRP, procalcitonin (PCT), serum amyloid A (SAA), ferritin, etc. Among these, CRP, PCT and ESR are the most commonly used parameters for assessing inflammation [6, 7].
Proinflammatory cytokines may be secreted in response to pathogen contact, tissue damage or psychosocial stressors, and mediate communication between the immune system and the brain [8]. Increases in proinflammatory cytokines are usually accommodational, temporary and regulated by anti-inflammatory processes. On the other hand, if an individual is receiving chronic cytokine treatment, or chronically exposed to disease or stress (chronic or unbalanced inflammatory response), inflammation and cytokines may lead to psychiatric disorders, such as behavioral symptoms, depression and anxiety. A broad range of studies have suggested that inflammation and proinflammatory cytokines might have a role in etiopathogenesis of major depressive disorder (MDD) [1, 4, 8–11]. Cytokines induce depressive symptoms through neuroendocrine processes, the hypothalamic-pituitary-adrenal (HPA) axis, neurotransmitter metabolism, synaptic plasticity, and neural circuits that regulate hippocampal neurogenesis, mood, motor activity, motivation, anxiety and the alarm system [1, 3, 12–14]. By causing desensitization of glucocorticoid receptors, cytokines disable the negative feedback mechanism acting on the HPA axis, which in turn leads to strong stimulation of HPA axis activity. While the response to acute stress is activation of the HPA axis, long-term complications arise when stress becomes chronic [11, 13, 15]. Cytokines also alter the metabolism of monoamines, most notably serotonin [8, 16]. In summation, findings that suggest levels of proinflammatory cytokines and APRs increase in MDD have been accumulating in recent years. Concurrent with ameliorating symptoms of depression, antidepressants help restore proinflammatory cytokine levels back to normal. It has been reported that selective serotonin reuptake inhibitors (SSRIs) in particular have regulatory effects on cytokine levels [17].
In this study, blood serum levels of the proinflammatory cytokines IL-1, IL-2 and IL-6, as well as the APRs ESH, haptoglobin and CRP were measured in healthy volunteers, patients diagnosed with MDD and patients with MDD after eight weeks of antidepressant (SSRI) treatment. The resulting data were analyzed in order to investigate the relationship between depression and neuroinflammation.
MATERIALS AND METHODS
Included in our study were 28 patients that visited the Psychiatry Clinic of Antalya Training and Research Hospital in consecutive order, were diagnosed with MDD according to the DSM-5 diagnostic criteria and completed the study (18 patients that did not attend the second examination and 16 patients that did not follow through the 8-week treatment plan were excluded), and 22 healthy individuals that consented to participate as the control group. The study protocol was approved by the Ethical Committee of Antalya Training and Research Hospital (approval number: 2019-153). A sociodemographic data form and the Structured Clinical Interview for DSM-5 form were filled out for each participant. The Hamilton Rating Scale for Depression (HRSD) was applied to patients diagnosed with depression once during their initial examination and once more at the end of the 8-week antidepressant treatment. Ten mL of venous blood was drawn from each participant, which were sent to the biochemistry laboratory for measuring levels of inflammatory markers. Additionally, following the 8‑week antidepressant treatment, 10 mL of venous blood was drawn from each study patient in order to measure levels of inflammatory markers.
The patient and control groups were compared with regards to age and gender. SSRI class of antidepressants were used for treating MDD patients; namely, sertraline (n = 10, 50–100 mg/day), escitalopram (n = 10, 10–20 mg/day) and paroxetine (n = 8, 20–40 mg/day). The criterion for response to treatment was determined as at least 50% decrease in HRSD score compared to the initial assessment. Criteria for exclusion from the study were as follows: illiteracy, mental retardation, internal diseases severe enough to deteriorate general health, age below 18, existing diagnosis of depression types other than MDD (psychotic, catatonic, atypical, melancholic, etc.), concurrent psychiatric disorders, pregnancy, acute infections within the last month or chronic infections, presence of autoimmune, allergic, neoplastic or endocrine disorders (thyroid or other endocrine dysfunctions), and intake of any psychoactive medication in the six weeks prior to the study. Exclusion criteria for healthy volunteers were as follows: existing diagnosis of any psychiatric disorder, cognitive issues that impede comprehension of the tests, illiteracy and age below 18.
Biochemical analysis. Samples of 10 mL of venous blood drawn from the participants were centrifuged at 3000 rpm for 10 min. The resulting serum samples were used for measuring levels of IL-1, IL-2, IL-6, haptoglobin and CRP. ELISA kits (YLbiont, Shanghai YL Biotech Co. Ltd., Shanghai, PRC) were used for measurement of IL-1, IL-2, IL-6 and haptoglobin levels. CRP levels were quantified with an automated AU-5800 clinical chemistry analyzer (Beckman Coulter Inc., Brea CA, USA). Sedimentation was assessed using 5 mL of total blood.
Statistical analysis. Descriptive statistics were given as mean ± standard deviation. Differences between means were deemed statistically significant if the P value was smaller than 0.05. Homogeneity of gender between the patient and control groups was assessed using the chi-squared test, while the independent samples t-test was utilized for comparing means of age and inflammatory marker levels between the groups. Differences between inflammatory marker levels in the patient group before and after treatment were analyzed using the paired samples t-test and the Wilcoxon signed-rank test. The paired samples t-test was used for the IL-6, haptoglobin, CRP and ESR parameters, while the Wilcoxon signed-rank test was used for the IL-1 and IL-2 parameters (depending on whether the parametric test assumptions were met or not). Variation in HRSD scores before and after treatment was assessed with the Wilcoxon signed-rank test.
Statistical analyses were performed using the software package SPSS Statistics for Windows, version 21.0. (IBM Corp., New York, USA).
RESULTS
A total of 28 MDD patients (68% female) were included in our study and their mean age was 42.64 ± 2.10 years. The control group consisted of 22 healthy individuals (64% female) with a mean age of 43.22 ± 2.44 years. There was no statistically significant difference between the patient and control groups in terms of age and gender (P > 0.05). Sociodemographic data of both groups are given in Table 1.
Serum cytokine and APR levels in the patient and control groups are shown in Table 2. Levels of IL-1, IL-2, IL-6 and haptoglobin were significantly different between the groups. No statistically significant difference was observed in levels of CRP or ESR.
Pre- and post-treatment serum cytokine and APR levels in the patient group are given in Table 3. Statistically significant differences were present between levels of IL-1, IL-2, IL-6 and haptoglobin, whereas CRP levels and ASR were not significantly different.
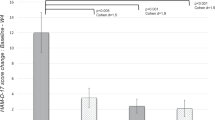
Mean HRSD score of the patients before antidepressant treatment was 22.92 ± 0.95, while that after the treatment was 9.67 ± 0.54. The reduction in post-treatment HRSD scores compared to pre-treatment scores, which was 58%, was statistically significant (P < 0.05). All participants in the patient group responded to the antidepressant treatment.
DISCUSSION AND CONCLUSIONS
In this study, we investigated whether serum levels of inflammatory mediators such as IL-1, IL-2, IL-6, haptoglobin, ESR and CRP significantly differ between MDD patients and healthy individuals; and whether said serum levels were altered by antidepressant treatment compared to pre-treatment levels. Our results showed that serum levels of proinflammatory cytokines (IL-1, IL-2 and IL-6) and haptoglobin were significantly higher in MDD patients than those in healthy individuals, while ESR and CRP levels were not significantly different between the two groups. When we compared inflammatory marker levels of MDD patients measured during the initial examination with those after the 8-week antidepressant treatment, levels of IL-1, IL-2, IL-6 and haptoglobin were significantly lower following treatment, whereas variation in ESR and CRP levels were not statistically significant.
In order to assess cytokine levels in MDD, Howren et al. carried out a meta-analysis of studies conducted between January 1967 and January 2008 [18]. Their analysis involved clinical sample and population-based studies that examined levels of CRP, IL-1 and IL-6. The results of this study indicate a positive correlation between major depression and levels of CRP, IL-1 and IL-6. Another meta-analysis performed by Dowlati et al. inspected studies carried out between June 1960 and August 2009, in which the study groups comprised of patients diagnosed with depression according to DSM-III-R or DSM-IV, but not treated with antidepressants [19]. A total of 136 studies were found between said dates, 24 of which were included in the meta-analysis. According to this meta-analysis, levels of TNF-α (13 studies, 438 patients, 350 healthy controls) and IL-6 (16 studies, 492 patients, 400 healthy controls) were higher in depression patients compared to healthy individuals. On the other hand, there was no statistically significant difference between patients and healthy individuals in levels of IL-1β (9 studies, 267 patients, 246 healthy controls), IL-2 (5 studies, 154 patients, 132 healthy controls), IL-4 (5 studies, 153 patients, 139 healthy controls), IL-8 (4 studies, 205 patients, 177 healthy controls), IL-10 (6 studies, 171 patients, 200 healthy controls) or INF-γ (4 studies, 131 patients, 107 healthy controls). Consequently, these results showed that levels of TNF-α and IL-6 were increased in MDD, which was interpreted as proof that the immune system is activated in cases of depression. While there are several published papers involving IL-1, IL-2, IL-6 and CRP, research on haptoglobin and ESR is limited. The few existing studies conducted by Maes et al., Chavda et al. and Vargas et al. indicated that depression significantly correlated with ESR and CRP levels [20–22].
Moreover, some clinical studies suggest that antidepressants contribute to normalization of the patient by lowering levels of serum proinflammatory cytokines, which are elevated pre-treatment. It has been alleged that especially SSRIs have regulatory effects on cytokines [17, 23]. In agreement, our study showed that following eight weeks of treatment with SSRI class of antidepressants, there was statistically significant reduction in inflammatory mediator levels.
In contrast, there are studies that have reported no relation between depression and cytokines [19, 24, 25]. In a study by Hocaoglu et al. that compared 30 depression patients and 30 healthy volunteers, no significant difference was observed in levels of IL-1β, IL-6, IL-8, IL-10, TNF-α or INF-γ between the two groups [25]. Similarly, there are numerous studies that suggest no significant relation exists between depression and inflammatory mediators. The results of our study indicate that an inflammatory response is active in the course of MDD and that levels of the inflammatory cytokines IL-1, IL-2 and IL-6, as well as the APR haptoglobin are elevated significantly. The other APRs investigated in our study, ESR and CPR, were not significantly different in depression patients compared to healthy individuals.
The notable feature of our study was that the proinflammatory cytokines IL-1, IL-2 and IL-6 were examined together with the APRs haptoglobin, ESR and CRP in MDD patients. Furthermore, comparison of these parameters pre- and post-treatment provides an important layer to the study.
The limitation of our study was that the number of participating patients and healthy individuals was low. We expect that future studies involving more participants will contribute significantly to the understanding of depression and the field of psychoimmunology.
REFERENCES
Capuron, L., and Miller, A.H., Pharmacol. Ther., 2011, vol. 130, no. 2, pp. 226–238.
Raison CL, Cowles MK, and Miller AH., Kaplan&Sadock’s Comprehensive Textbook of Psychiatry, 9th edition, 2009, pp. 175–197.
Tuğlu C., and Kara S.H., Klin. Psikofarmakol. Bülteni (Bull. Clin. Psychopharmacol.), 2003, vol. 13, no. 3, pp. 142–150
Dinan, T.G., Curr. Opin. Psychiatry, 2009, vol. 22, no. 1, pp. 32–36.
Seidel, A., Arolt, V., Hunstiger, M., Rink, L., Behnisch, A., and Kirchner, H., Scand. J. Immunol., 1995, vol. 41, no. 6, pp. 534–538.
Kushner, I., Ann. NY Acad. Sci., 1982, vol. 389, pp. 39–48.
Gabay, C., and Kushner, I., N. Engl. J. Med., 1999, vol. 340, no. 6, pp. 448–454.
Schiepers, O.J., Wichers, M.C., and Maes, M., Prog. Neuropsychopharmacol. Biol. Psychiatry, 2005, vol. 29, no. 2, pp. 201–217.
Gümrü, S., and Arıcıoğlu, F., Journal of Marmara University Institute of Health Sciences, 2012, vol. 2, no. 3, pp. 103–107.
Krishnadas, R. and Cavanagh, J., J. Neurol. Neurosurg. Psychiatry, 2012, vol. 83, no. 5, pp. 495–502.
Raedler, T.J., Curr. Opin. Psychiatry, 2011, vol. 24, no. 6, pp. 519–525.
Doksat, M.K., Klin. Psikofarmakol. Bul., 2003, vol. 13, pp. 97–108.
Miller, A.H., Maletic, V., and Raison, C.L., Biol. Psychiatry, 2009, vol. 65, no. 9, pp. 732–741.
Jones, K.A. and Thomsen, C., Mol. Cell. Neurosci., 2013, vol. 53, pp. 52–62.
Doksat, K. and Savrun, M., Yeni Symposium, 2002, vol. 40, pp. 90–99.
Maes, M., Ringel, K., Kubera, M., Berk, M., and Rybakowski, J., J. Affect Disord., 2012, vol. 136, no. 3, pp. 386–392.
Hannestad, J., DellaGioia, N., and Bloch, M., Neuropsychopharmacology, 2011, vol. 36, no. 12, pp. 2452–2459.
Howren, M.B., Lamkin, D.M., and Suls, J., Psychosom. Med., 2009, vol. 71, no. 2, pp. 171–186.
Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E. K., and Lanctôt, K. L., Biol. Psychiatry, 2010, vol. 67, no. 5, pp. 446–457.
Maes, M., Scharp, S., Grootel, V.L., Uyttenbroeck, W., Cooreman, W., Cosyns, P., and Suy, E., J. Affect. Disord., 1992, vol. 24, no. 3, pp. 183–192.
Chavda, N., Kantharia, N.D., Jaykaran, J. Pharmacol. Pharmacother., 2011, vol. 2, no. 1, pp. 11–16.
Vargas, H.O., Nunes, S.O.V., de Castro, M.R.P., Vargas, M.M., Barbosa, D.S., Bortolasci, C.C., Venugopal, K., Dodd, S., and Berk, M., Neurosci. Lett., 2013, vol. 544, pp. 136–140.
Janssen, D.G., Caniato, R.N., Verster, J.C., and Baune, B.T., Hum. Psychopharmacol., 2010, vol. 25, no. 3, pp. 201–215.
Kocabaşoğlu, N., Konuk, N., Öztürk, S., and Bayar, R., Yeni Symposium, 2000, vol. 36, pp. 83–87.
Hocaoglu, C., Kural, B., Aliyazıcıoglu, R., Deger, O., and Cengiz, S., Metab. Brain Dis., 2012, vol. 27, no. 4, pp. 425–430.
ACKNOWLEDGMENTS
The authors thank Dr. Alper Sınan who helped in the statistical analysis of the study. Antalya Training and Research Hospital Ethics Committee Provided the kits of cytokines (IL-1,IL-2,IL-6) and acute phase reactants (Haptoglobin, CRP, ESR) that we use in the biochemistry laboratory for our study.
Funding
No external funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Ethical approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Korkut, S., Kulaksizoglu, S. The Relationship of Antidepressant Therapy with Neuroinflammatory Changes and Inflammatory Response in Major Depressive Disorder. Neurochem. J. 15, 477–481 (2021). https://doi.org/10.1134/S1819712421040061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1819712421040061