Abstract
Crosslinked polysiloxanes with boron bis(dibenzoylmethanate) complexes used as crosslink junctions of polymer networks are first synthesized, and their physicochemical, mechanical, thermal, and fluorescent properties are studied. It is shown that the polymers under study feature the elastic behavior, possess high thermal and thermo-oxidative stability, and exhibit intense fluorescence in a wide wavelength range (400‒700 nm).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In two recent decades, materials possessing luminescent properties have been very promising objects of research in polymer chemistry and materials science, since they are used in organic electronics, sensorics, biomedicine, and other fields of science and engineering [1–3]. Luminophores here may be organic dyes, quantum dots, metal complexes, etc. [4‒7].
At present, one of the most popular classes of organic fluorophores is various boron-containing complexes. Owing to their valuable photophysical properties these complexes are in wide use in the development of new fluorescent materials [8–10] demonstrating unique optical [11, 12], mechanochromic [13–19], photochromic [20, 21], and sensor characteristics [22–26].
Among a wide range of known fluorescent boron complexes, ionic complexes, such as boron bis(β-diketonates), should be mentioned [27, 28]. Discovered in the mid-20th century these complexes prepared by the interaction of β-diketones with boron trichloride [29, 30] have been still insufficiently studied and up to now trigger the interest of researchers. Specifically, this is due to the fact that boron bis(β-diketonates) have a high fluorescence extinction coefficient in a wide wavelength range and many other characteristics having high potential for application [31]. For example, bis(β-diketone) boron complexes based on curcumin derivatives are used as targeted [32] or intermediate compounds in the synthesis of biologically active curcumins [33, 34]. Silica-based mesostructured thin films modified with dibenzoylmethane can be employed as selective sensors for BCl3 and BF3 detection in the gaseous state [35]. Boron bis(β-diketonates) with aliphatic substituents at ligands demonstrate strong thermochromic properties in the temperature range from 25 to ‒196°С [36]. Boron avobenzone complexes exhibit solvatochromic fluorescence in solution, and in the solid state the emission of the complexes changes color in the presence of water vapor [37].
It is known that siloxanes of various structure are actively used as matrices for preparing new luminescent materials [6, 38–45]. This is due to their high thermal stability, flexibility, elasticity, low toxicity, biocompatibility, and hydrophobicity. As was shown in [46, 47], polysiloxanes modified with boron or europium β-diketonates offer promise as new luminescent materials with sensor properties [46, 47].
This study addresses the synthesis of new fluorescent materials from polysiloxanes crosslinked via interaction between β-diketone fragments distributed along the polymer chain and boron trichloride accompanied by formation of the fluorescent complex and characterization of their mechanical, thermal, and fluorescent properties.
EXPERIMENTAL
Materials and Methods
Polysiloxanes 1a and 1b were prepared according to [48]. Boron trichloride (1 mol/L solution in hexane) and dibenzoylmethane were obtained commercially from ABCR, and boron bis(dibenzoylmethanate) complex 4 was prepared according to the technique described in [31]. All reactants were used as received. Solvents were purified before use as follows: tetrahydrofuran was dried and distilled over CaH2; dichloromethane, over P2O5.
1H NMR spectra were recorded on a Bruker Avance II spectrometer (400 MHz). Chemical shifts are given relative to the signal of chloroform (δ = 7.25 ppm).
IR spectra were recorded on a Shimadzu IRTracer-100 FTIR spectrometer using samples prepared as KBr pellets and films.
Molecular weight characteristics of polymers were analyzed by GPC using a Shimadzu Prominent System chromatograph equipped with a RID-20A detector in toluene (1 mL/min). A Phenogel 105 Å GPC column was calibrated against polystyrene standards.
Thermogravimetric analysis was performed on a Shimadzu DTG-60H synchronous thermal analyzer using ~10 mg samples at a heating rate of 10°С/min in air and argon. Temperature, at which the 5% weight loss was detected, was taken as the degradation onset temperature.
Differential scanning calorimetry was performed on a Mettler-Toledo DSC-3 instrument at a heating rate of 10°С/min under argon.
The mechanical properties of the samples were determined on a LLOYD Instruments LR5K Plus tensile testing machine at a strain rate of 100 mm/min.
The efficiency of formation of crosslinked networks was estimated by analyzing gel fractions of the samples dried to a constant weight in a vacuum oven (1 mbar, Т = 80°C). The noncrosslinked oligomers and precursors were then extracted by tetrahydrofuran in a Soxhlet apparatus for 14 h. Upon extraction, the samples were dried in the vacuum oven under the same conditions to a constant weight. The gel fraction of the samples was calculated according to the following equation:
where W0 and W1 is the weight of the dried sample before and after extraction, respectively.
Absorption spectra were measured on a Shimadzu UV-1900 spectrophotometer; fluorescence spectra, on a Shimadzu RF-6000 spectrofluorometer. The deaerated solution of 9,10-diphenylanthracene in cyclohexane (Фf = 0.9) was used as a standard to estimate fluorescence quantum yield.
Fluorescence decay kinetics was monitored on a Picoquant Fluotime 300 time-resolved fluorescence spectrometer. Laser LDH-D-C-375 was used as an excitation source (λex = 375 nm). In short lifetime measurements up to 10 µs the instrument operated in the time-correlated single photon counting (TCSPC) mode. If lifetimes were above 10 µs, the instrument operated in the multi-channel scaling (MCS) mode.
Excitation can be induced by not only single pulses but also by a pulse “burst.” The pulse repetition rate is chosen to be maximum possible. The “burst” repetition rate can be also set, and the flash duration can be specified by controlling the number of pulses in the “burst.” The shape of the front for such a flash will be determined by characteristics of the single pulse. For the used system the width at half height is ~40 ps.
The data obtained were approximated using the Easytau2 software from Picoquant in terms of the multiexponential model.
Synthesis of Compounds 3a and 3b
To a solution of polymer 1а (408 mg, 0.34 mmol) or 1b (578 mg, 0.193 mmol) in dry THF (10 mL) at room temperature, a solution of BCl3 in hexane with a concentration of 1 mol/L (175 µL for 3a and 96.7 µL for 3b) was added. The reaction mixture was intensively stirred for several seconds, and the formed gel was poured on a Teflon plate under inert atmosphere. Upon complete solvent removal and film formation the sample were kept in the vacuum oven (1 mbar, Т = 80°С) for 8 h. The crosslinked polymers were obtained in the form of light-brown (3a) and light-green (3b) films. For 3а: IR spectrum (KBr, cm‒1): 2963, 2921, 2852, 1609, 1538, 1413, 1262, 1097, 1019, 864, 801, 688, 661; for 3b: IR spectrum (KBr, cm‒1): 2963, 2905, 1609, 1541, 1490, 1413, 1262, 1098, 1018, 862, 801, 689, 662.
RESULTS AND DISCUSSION
Synthesis
Polydimethylsiloxanes crosslinked via formation of borate complexes were synthesized from polydimethylsiloxanes 1a and 1b containing β-diketonate fragments distributed along the chain:

Polymers 1a and 1b were synthesized as described in [48]. To determine effect of the degree of crosslinking on the properties of materials compounds with various content of dibenzoylmethane fragments in the polymer chain were prepared. The molecular weight characteristics of compounds 1a and 1b are listed in Table 1, and their GPC curves are shown in Fig. 1.
Crosslinked polydimethylsiloxanes 3a and 3b were prepared by the reaction of boron trichloride with β‑diketone-containing polymers 1a and 1b under inert atmosphere as follows. To solutions of polymers 1a and 1b in THF a boron trichloride solution in hexane was added under inert atmosphere. The gel resulting from the short-term stirring was poured on the Teflon plate and allowed to stay under inert atmosphere until complete evaporation of solvents was attained, and the samples were dried to a constant weight. Thus, two films 3a and 3b with different degrees of crosslinking were obtained. Crosslink junctions in these materials were complexes of [(DBM)2B]+ with Cl‒ anion as a counterion. The photographs of the films are presented in Fig. 2.
The IR spectra (Fig. 3) show characteristic bands corresponding to vibrations of C=O bonds of coordinated β-diketone groups in the range of 1609‒1414 cm‒1. Characteristic bands related to vibrations of Si‒C bonds are seen at 1262 and 864 cm‒1. Intense bands observed in the range of 1098‒1018 cm‒1 and at 801 cm‒1 are due to the asymmetric and symmetric vibrations of Si‒O‒Si bonds, respectively. Bands in the range of 2963‒2095 cm‒1 correspond to vibrations of С‒Н bonds.
To evaluate the efficiency of formation of crosslinked structures the gel fractions were analyzed. Analysis was performed by extracting the samples by tetrahydrofuran in the Soxhlet apparatus. The gel fraction of polymers 3a and 3b was 59 and 8%, respectively, which suggests a high degree of crosslinking in the case of polydimethylsiloxane containing a larger number of grafted β-diketone fragments.
Mechanical Properties
Figure 4 shows the stress–strain curves recorded during the uniaxial stretching of the samples. The results of the analysis of the gel fractions of the samples are consistent with the mechanical testing: polymer 3а having a denser spatial network features better strength characteristics compared with polymer 3b.
The tensile strength for polymer 3а was 0.33 MPa and the Young modulus was 0.44 MPa, which indicates a higher strength of this material. At the same time, polymer 3b is more elastic, since its modulus is lower and elongation at break is higher (140%). The mechanical properties of the compounds are summarized in Table 2.
Thermal Properties
The thermal stability of the samples was studied by the TGA method in the temperature range from 50 to 800°С in air and argon. The data shown in Figs. 5a and 5b and Table 3 indicate that the tested materials possess a high thermal and thermo-oxidative stability, since the onset degradation temperature of the samples is in the range of 300‒400°С. Polymer 3b is more stable than polymer 3а which is most likely related to a smaller content of borate complexes in it. Note that the percent of the solid residue recovered upon the thermo-oxidative degradation of the samples is small. It is probable that boron complexes contained in the polymers may catalyze the rupture of siloxane bonds, thus leading to the production of a larger amount of volatiles during analysis.
The DSC curves of compounds 3a and 3b (Fig. 5c) in the temperature range from ‒145 to 200°С show only glass transition of the samples at ‒112 and ‒127°С, respectively. A decreased glass transition temperature of compound 3а may be due to sample structuring or a high content of the complexes. The introduction of complex (DBM)2B+Cl‒ into the polymer contributes to the suppression of crystallization ability typical for polydimethylsiloxanes.
Optical Properties
The optical properties of polymers 3a and 3b were studied by steady-state and time-resolved fluorescence spectroscopy. To examine the optical properties in detail model compound 5 was prepared from dibenzoylmethane and boron trichloride according to the technique described in [31]:

The absorption and emission spectra of compound 5 are presented in Fig. 6. The absorption spectrum of compound 5 in dichloromethane exhibits three well-defined bands with a maximum at 400 nm, and the molar extinction coefficient is 62 500 М−1 cm−1. The emission spectrum of compound 5 in dichloromethane shows two bands at 412 nm (maximum) and 433 nm and a shoulder at 463 nm. The fluorescence quantum yield of the complex is 80%, and its lifetime is 1.27 ns.
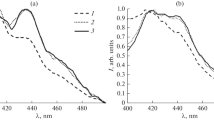
An examination of the fluorescence spectra of polymers 3a and 3b (Fig. 7а) shows that the shape of the spectra changes depending on the fluorescence excitation wavelength. For example, under excitation of polymer 3a at 400 nm a broad structureless fluorescence band with a maximum at 530 nm is observed in the range of 400‒700 nm which is typical for the emission of excimers of these complexes formed from aggregates in the excited state. Under excitation of polymer 3a at 370 nm a shoulder appears in the fluorescence spectrum at 430 nm, as is typical for the emission of the monomer complex. A more pronounced picture can be seen for polymer 3b containing a smaller amount of borate complexes. In the fluorescence spectra of this polymer under excitation at wavelengths of 370 and 400 nm a considerable fraction of emission of the monomer with a maximum at ~420 nm is observed. The fluorescence excitation spectra of polymers 3a and 3b in the condensed state are presented in Fig. 7b.
Figure 8 shows the luminescence decay kinetics of polymers 3a and 3b in the condensed state measured at wavelengths of 433 and 540 nm. The luminescence decay kinetics of the solid samples is nonmonoexponential. The results of approximation by models containing the minimal amount of exponential terms, for which χ2 is in the range of 0.8‒1.3 [49] and the distribution of discrepancies and autocorrelation function are uniform, are presented in Table 4. With a decrease in the number of exponential terms in the model by unity for the kinetic curves measured for 3а and 3b at 433 nm and for 3b at 540 nm χ2 increases to 1.6. For the kinetic curve measured for 3а at 540 nm χ2 grows to 1.32 and a considerable nonhomogeneity of discrepancies and autocorrelation function oscillations are observed. At a wavelength of 433 nm the kinetics is in the nanosecond range with an average lifetime of ~1 ns for both samples (Fig. 8a). The kinetic characteristics of luminescence decay measured at a wavelength of 540 nm are much longer: the average lifetime is 28 ns for sample 3а (Fig. 8b) and 1370 ns for sample 3b (Fig. 8c). A decrease in the average lifetime with an increase in the amount of fluorophores apparently suggests that the role of excited state loss processes becomes more substantial with increasing degree of aggregation. It can be assumed that with increasing aggregate size a distance, over which the migration of excitation energy is possible, also increases. As a result, the probability of excitation losses on various impurity traps grows. An alternative explanation is that rate of intercombination conversion in aggregates increases [50]. This issue calls for further studies.
Luminescence decay kinetic curves (marked by black and blue) for (a) 3a and 3b at 433 nm, (b) 3а at 540 nm, (c) 3b at 540 nm measured in the TCSPC mode, and (d) 3b at 540 nm measured in the MCS mode at a flash duration of 6 µs. The excitation wavelength is 375 nm. Instrumental response functions (IRF) measured at 375 nm in the TCSPC mode are marked by red. Color figures are available in the electronic version.
Under irradiation of polymer 3b luminescence intensity reaches the stationary value ~6 μs which is associated with the accumulation of long-lived, presumably, triplet states in the sample. At an excitation flash duration of 6 µs for sample 3b luminescence with an average lifetime of 4.6 µs is observed (Fig. 8d). By analogy with polydimethylsiloxanes containing fragments of boron difluoride diketonates it can be assumed that the presence of the fluorescence band with a maximum at 540 nm and the lifetime of tens of nanoseconds are related to the aggregation of fluorophore fragments and excimer fluorescence characteristic of aggregates [46]. The appearance of long-term luminescence with microsecond decay times is most probably associated with the mechanism of thermally activated delayed fluorescence of aggregates, which is also typical for the aggregates of boron diketone complexes [50–52].
CONCLUSIONS
The synthesis of crosslinked polysiloxanes via the reaction of grafted β-diketone ligands with boron trichloride accompanied by formation of the fluorescent borate complex [(DBM)2B]+Cl‒ was first studied. The mechanical properties of the synthesized polymers were investigated; the data obtained demonstrated that the materials feature elasticity (ε = 120‒140%) with a low tensile strength: 0.33 MPa for polymer 3a and 0.19 MPa for polymer 3b. The elastic moduli of the tested compounds are 0.44 MPa for 3a and 0.26 MPa for 3b. It is shown that these compounds possess high thermal and thermo-oxidative stability and their glass transition temperatures are ‒112 and ‒127°С for polymers 3a and 3b, respectively. Furthermore, they are incapable of crystallization typical for polysiloxanes. The study of the optical properties revealed that in the condensed state polymers 3a and 3b exhibit intense fluorescence in a wide wavelength range (400‒700 nm) with a maximum at 530 nm, as is characteristic of the emission of excimers of boron complexes resulting from the aggregates in the excited state.
REFERENCES
S. Wei, Z. Li, W. Lu, H. Liu, J. Zhang, T. Chen, and B. Z. Tang, Angew. Chemie Int. Ed. 60, 8608 (2021).
G. Ahumada and M. Borkowska, Polymers (Basel) 14, 1118 (2022).
S. Cichosz, A. Masek, and M. Zaborski, Polym. Test. 67, 342 (2018).
C. Calvino, A. Guha, C. Weder, and S. Schrett, Adv. Mater. 30, 1704603 (2018).
X. Du, C. Wang, G. Wu, and S. Chen, Angew. Chemie Int. Ed. 60, 8585 (2021).
L. Birchall, A. Foerster, G. A. Rance, A. Terry, R. D. Wildman, and C. J. Tuck, Sens. Actuators, A 347, 113977 (2022).
J. Alday, A. Mazzeo, and S. Suarez, Inorg. Chim. Acta 510, 119696 (2020).
P.-Z. Chen, L.-Y. Niu, Y.-Z. Chen, and Q.-Z. Yang, Coord. Chem. Rev. 350, 196 (2017).
K. Tanaka and Y. Chujo, NPG Asia Mater. 7, e223 (2015).
A. Loudet and K. Burgess, Chem. Rev. 107, 4891 (2007).
D. Frath, J. Massue, G. Ulrich, and R. Ziessel, Angew. Chemie Int. Ed. 53, 2290 (2014).
D. Li, H. Zhang, and Y. Wang, Chem. Soc. Rev. 42, 8416 (2013).
R. Yoshii, K. Suenaga, K. Tanaka, and Y. Chujo, Chem.-Eur. J. 21, 7231 (2015).
T. Sagawa, F. Ito, A. Sakai, Y. Ogata, K. Tanaka, and H. Ikeda, Photochem. Photobiol. Sci. 15, 420 (2016).
Y. N. Kononevich, M. N. Temnikov, A. A. Korlyukov, A. D. Volodin, P. V. Dorovatovskii, V. A. Sazhnikov, A. A. Safonov, D. S. Ionov, A. A. Ivanov, N. M. Surin, E. A. Svidchenko, and A. M. Muzafarov, ChemPlusChem 85, 1111 (2020).
W. A. Morris, M. Kolpaczynska, and C. L. Fraser, J. Phys. Chem. 120, 22539 (2016).
M. Louis, C. Piñero García, A. Brosseau, C. Allain, and R. Metivier, J. Phys. Chem. Lett. 10, 4758 (2019).
L. Zhang, L.-L. Ma, X. Wang, and X.-Y. Zhao, J. Lumin. 214, 116560 (2019).
M. Louis, R. Sethy, J. Kumar, S. Katao, R. Guillot, T. Nakashima, C. Allain, T. Kawai, and R. Metivier, Chem. Sci. 10, 843 (2019).
C.-T. Poon, W. H. Lam, H.-L. Wong, and V. W.-W. Yam, J. Am. Chem. Soc. 132, 13992 (2010).
Z. Li, D. Wang, D. Ramella, H. Gao, H. Cao, Y. Zhao, Z. Miao, Z. Yang, and W. He, Polym. Chem. 11, 3046 (2020).
M. Zhuang, S. Joshi, H. Sun, T. Batabyal, C. L. Fraser, and J. Kapur, Sci. Rep. 11, 1076 (2021).
Z. Li, Y. Pei, S. Hou, Y. Dai, D. Liu, J. Zhu, Y.-P. Zhu, and X. Liu, Dyes Pigm. 179, 108419 (2020).
Y. N. Kononevich, V. A. Sazhnikov, A. S. Belova, A. A. Korlyukov, A. D. Volodin, A. A. Safonov, G. A. Yurasik, D. S. Ionov, and A. M. Muzafarov, New J. Chem. 43, 13725 (2019).
D. Ionov, G. Yurasik, Y. Kononevich, V. Sazhnikov, A. Muzafarov, and M. Alfimov, Procedia Eng. 168, 341 (2016).
D. S. Ionov, V. A. Sazhnikov, G. A. Yurasik, A. V. Antonov, Y. N. Kononevich, and M. V. Alfimov, High Energy Chem. 49, 183 (2015).
N. M. D. Brown and P. Bladon, J. Chem. Soc. A, No. 526, 526 (1969).
G. A. Reynolds and C. H. Chen, J. Heterocycl. Chem. 22, 657 (1985).
D. Martin, Chem. Rev. 34, 461 (1944).
W. Gerrard and M. F. Lappert, Chem. Rev. 58, 1081 (1958).
A. Barabas, E. Isfan, M. Roman, M. Paraschiv, E. Romas, and A. T. Balaban, Tetrahedron 24, 1133 (1968).
Z. Sui, R. Salto, J. Li, C. Craik, and P. R. Ortiz de Montellano, Bioorg. Med. Chem. 1, 415 (1993).
W.-Y. Shao, Y.-N. Cao, Z.-W. Yu, W.-J. Pan, X. Qiu, X.-Z. Bu, L.-K. An, Z.-S. Huang, L.-Q. Gu, and A. S. C. Chan, Tetrahedron Lett. 47, 4085 (2006).
L. Shi, L. Gao, S. Cai, Q. Xiong, and Z. Ma, Eur. J. Med. Chem. 221, 113528 (2021).
P. Banet, L. Legagneux, P. Hesemann, J. Moreau, L. Nicole, A. Quach, C. Sanchez, and T. Tranthi, Sens. Actuators, B 130, 1 (2008).
X. Chen, X. Zhang, and G. Zhang, Chem. Commun. 51, 161 (2015).
X. Zhang and G. Zhang, Anal. Methods 4, 2641 (2012).
Y. Liang, L. Xu, F. Qu, K. Tang, H. Wang, and W. W. Yu, Polym. Chem. 10, 4818 (2019).
I. Toulokhonova, B. Bjerke-Kroll, and R. West, J. Organomet. Chem. 686, 101 (2003).
A. C. Benniston, G. Copley, A. Harriman, and R. Ryan, J. Mater. Chem. 21, 2601 (2011).
W. Kasprzyk, P. Krzywda, S. Bednarz, and D. Bogdal, RSC Adv. 5, 90473 (2015).
J. Chen, L. Song, Y. Wu, B. Zhao, and J. Deng, ACS Appl. Polym. Mater. 4, 4264 (2022).
A. A. Pakhomov, Yu. N. Kononevich, M. V. Stukalova, E. A. Svidchenko, N. M. Surin, G. V. Cherkaev, O. I. Shchegolikhina, V. I. Martynov, and A. M. Muzafarov, Tetrahedron Lett. 57, 979 (2016).
A. A. Pakhomov, V. B. Mironiuk, Yu. N. Kononevich, A. A. Korlyukov, A. D. Volodin, T. A. Pryakhina, V. I. Martynov, and A. M. Muzafarov, Mendeleev Commun. 27, 363 (2017).
A. A. Pakhomov, E. E. Kim, Yu. N. Kononevich, D. S. Ionov, M. A. Maksimova, V. B. Khalchenia, E. G. Maksimov, A. A. Anisimov, O. I. Shchegolikhina, V. I. Martynov, and A. M. Muzafarov, Dyes Pigm. 203, 110371 (2022).
A. S. Belova, A. G. Khchoyan, T. M. Il’ina, Yu. N. Kononevich, D. S. Ionov, V. A. Sazhnikov, D. A. Khanin, G. G. Nikiforova, V. G. Vasil’ev, and A. M. Muzafarov, Polymers (Basel) 14, 5075 (2022).
E. E. Kim, Yu. N. Kononevich, Y. S. Dyuzhikova, D. S. Ionov, D. A. Khanin, G. G. Nikiforova, O. I. Shchegolikhina, V. G. Vasil’ev, and A. M. Muzafarov, Polymers (Basel) 14, 2554 (2022).
E. E. Kim, Yu. N. Kononevich, A. A. Anisimov, M. I. Buzin, V. G. Vasil’ev, A. A. Korlyukov, D. S. Ionov, D. A. Khanin, E. V. Shtykova, V. V. Volkov, and A. M. Muzafarov, React. Funct. Polym. 164, 104896 (2021).
D. F. Eaton, Pure Appl. Chem. 62, 1631 (1990).
X. Sun, X. Wang, X. Li, J. Ge, Q. Zhang, J. Jiang, and G. Zhang, Macromol. Rapid Commun. 36, 298 (2015).
E. V. Fedorenko, M. K. Behra, and N Kanwishera, J. Fluoresc. 26, 1839 (2016).
A. A. Khrebtov, E. V. Fedorenko, and A. G. Mirochnik, Polymer (Guildf) 256, 125255 (2022).
Funding
This work was supported by the Russian Science Foundation (project no. 18-73-10152). Characterization of compounds by NMR spectroscopy, TGA, and DSC performed using equipment of the Center for Molecule Composition Studies at the Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences was supported by the Ministry of Science and Higher Education of the Russian Federation (contract no. 075-03-2023-642). Luminescence lifetimes were measured using equipment of the Center for Collective Use “Structural Diagnostics of Materials” within the framework of State Assignment for the Federal National Research Center “Crystallography and Photonics,” Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by T. Soboleva
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, E.E., Il’ina, T.M., Kononevich, Y.N. et al. New Fluorescent Materials Based on Polysiloxanes and Boron Bis(β-diketonates). Polym. Sci. Ser. C 65, 267–276 (2023). https://doi.org/10.1134/S1811238223700376
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1811238223700376