Abstract
The objective of this study was to assess the level of antibodies to carbamylated proteins and analyze the clinical and immunological associations in patients with ACPA-negative and ACPA-positive variants of rheumatoid arthritis.
Materials and methods
. The study involved 150 patients with a reliable diagnosis of rheumatoid arthritis and 25 patients as healthy controls. Depending on ACPA values, two groups of patients were recruited: ACPA-positive (n = 75) and ACPA-negative (n = 75). RA activity was assessed by the DAS28 index. Determination of antibodies to carbamylated proteins was performed by enzyme-linked immunosorbent assay (BlueGene Biotech, China). Quantitative determination of ACPA in serum was performed by enzyme immunoassay using a commercial reagent kit (AxisShield, UK; upper limit of normal 5.0 U/mL; Orgentec, Germany; upper limit of normal 20.0 U/mL).
Results and discussion
. Median anti-CarP in patients with RA was 126.2 [100.83; 157.41] ng/mL and was statistically significantly higher (p < 0.001) than in healthy controls (88.89 [70.53; 107.75] ng/mL). Among all patients with RA, 50 (33.3%) were anti-Carp-positive (22 (29.3%) in the ACPA(+) group and 28 (37.3%) in the ACPA(–) group), and one (2%) volunteer from healthy controls was anti-CarP(+) (p = 0.002). In ROC analysis performed to assess the diagnostic significance of anti-CarP for RA for all patients with RA, the area under the curve was 0.783 ± 0.047 with 95% CI: 0.691–0.874 (p < 0.001), with a cut-off point of 143 ng/mL, specificity 96%, sensitivity 36.7%.
In the ACPA(+) RA group, the erosion count was statistically significantly higher (p = 0.044) in anti-CarP(+) patients than in anti-CarP(–) patients. A weak direct correlation between anti-CarP and DAS28 was found in the ACPA(–) RA group.
Conclusions
. We studied the predictive value of anti-CarP as an auxiliary biomarker in ACPA(+) and ACPA(–) subtypes of RA. ACPA(+) anti-CarP(+) patients have a more “erosive” subtype of the disease than ACPA(+) anti-CarP(–) patients. In ACPA(–) patients, anti-CarP helps to identify a more erosive subtype of the disease, and among ACPA(–) patients it helps to reduce the proportion of seronegative patients. Further studies are required to determine the optimal standards for the laboratory diagnosis of anti-CarP and to clarify the diagnostic potential of these ABs as part of the differential diagnosis of arthritis in other rheumatic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rheumatoid arthritis (RA) is the most common immunoinflammatory (autoimmune) rheumatic disease (IRD), which manifests itself by chronic erosive arthritis and systemic damage to internal organs [1]. In the diagnosis of rheumatoid arthritis, the determination of specific biomarkers—rheumatoid factor (RF) IgM, anti-citrullinated protein antibodies (ACPA), and anti-mutated citrullinated vimentin antibodies (anti-MCV)—is of great importance. RF and ACPA are included in the ACR/EULAR 2010 criteria and, depending on their presence, seropositive and seronegative variants of RA are distinguished. Over the last decade, data on new highly sensitive and specific biomarkers of RA have been accumulated [2]. Antibodies to carbamylated proteins (anti-CarP) are currently recognized as one of the promising new biomarkers.
Carbamylation is a nonenzymatic posttranslational modification of a protein in which the reaction of cyanate with the ε-amino group of the lysine side chain results in the formation of homocitrulline [3]. It was established that, during inflammation, the production of myeloperoxidase by neutrophils stimulates carbamylation due to the oxidation of thiocyanate with hydrogen peroxide [4]. According to experimental studies, the development of an immune response to carbamylated proteins was accompanied by the synthesis of interferon-γ (IFN-γ), interleukin-10 (IL-10), and IL-17, as well as chemotaxis and proliferation of CD4+ T-lymphocytes associated with the development of erosive arthritis [5]. O’Neil et al. [6] found a correlation between the level of carbamylated histones in neutrophil extracellular traps (NETs) and osteoclastogenesis.
According to a meta-analysis [7], when comparing patients with RA and healthy controls, the sensitivity of anti-CarP determination was 42% and the specificity was 96%. Kuznetsova et al. [8] showed that antibodies to carbamylated vimentin were found in patients with RA more often than classical serological markers of RF and ACPA. It is known that anti-CarP-positive patients can be negative for RF and ACPA [3, 8–13]. Anti-CarP can be detected before the onset of RA and are considered as a predictor of disease development [9, 14]. The combination of anti-CarP with RF and ACPA increases the specificity of diagnosing RA relative to healthy controls [15].
There are data of the features of the anti-CarP-positive subtype of RA. Anti-CarP positive patients have more erosive arthritis according to radiography data, and this is more typical for the ACPA-negative patients [3, 9, 11, 12, 16–18]. Interstitial lung disease as a systemic manifestation of RA is also associated with an increase in anti-CarP [19, 20]. In a Spanish cohort of patients, it was shown that, among patients with anti-CarP, mortality was higher, mainly due to damage to the respiratory system [21].
The purpose of the study was to assess the level of antibodies to carbamylated proteins and analyze clinical and immunological associations in patients with ACPA-negative and ACPA-positive variants of rheumatoid arthritis.
MATERIALS AND METHODS
Patients with a reliable diagnosis of RA according to the 2010 ACR/EULAR criteria were recruited on the basis of the Nasonova Research Institute of Rheumatology. The study involved 150 patients after excluding diseases from the group of spondyloarthritis, microcrystalline arthritis, systemic lupus erythematosus, etc., and 25 healthy donors. Patients with psoriasis were not included in the study.
The majority of patients 128 (85.3%) were female, middle-aged, with a long course of the disease (Table 1). To assess RA activity, the DAS28 index was used, determined using ESR and CRP [22].
ESR was determined using the standard international Westergren method (norm ≤30 mm/h). Serum concentrations of CRP and RF IgM were measured using the immunonephelometric method on a BN ProSpec analyzer (Siemens, Germany). The upper limit of the norm for serum CRP was 5.0 mg/L. According to the manufacturer’s instructions, a concentration equal to 15.0 IU/mL was taken as the upper limit of the norm for RF IgM. Quantitative determination of ACPA in blood serum was performed by ELISA using commercial reagent kits (AxisShield, UK, upper limit of the norm 5.0 U/mL and Orgentec, Germany, upper limit of norm 20.0 U/mL). Antibodies to carbamylated proteins were determined by ELISA (BlueGene Biotech, China). Assessment of radiological changes in the joints of the hands and feet was carried out using the Sharp method as modified by van der Heijde.
The results were statistically processed using the IBM SPSS Statistics 26 software package, including the generally accepted methods of parametric and nonparametric analysis. When statistically processing data, quantitative variables were described using the arithmetic mean (M), standard deviation (δ), median (Me), and 25th and 75th percentiles. Qualitative variables were described by absolute and relative frequencies (percentages). For quantitative variables, a test for normality of distribution was performed. The results were evaluated using Pearson’s χ2 test (analysis of contingency tables) and unpaired Student’s t test. If the distribution differed from normal, the Mann–Whitney U test was used; when comparing three or more groups, the Kruskal–Wallis test was used. Correlation analysis was carried out using the Spearman method. ROC curves were constructed to describe the diagnostic characteristics of anti-CarP. Differences were considered statistically significant at p < 0.05.
RESULTS
Depending on the ACPA values, the patients were divided into two groups—ACPA-positive and ACPA-negative (Table 2). The ACPA(+) group included patients (n = 75) with ACPA values > 2 norms, and the ACPA(–) group included patients (n = 75) with values below the upper limit of the norm. There were no statistically significant differences in age, duration of the disease, and therapy with basic antiinflammatory genetically engineered biological and targeted synthetic drugs between the groups, so they were considered comparable in these parameters. In ACPA(+) patients, C-reactive protein values were higher, extra-articular manifestations were more common, and a higher number of erosions and radiological stages of RA were noted.
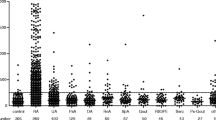
Median anti-CarP in patients with RA was 126.2 [100.83; 157.41] ng/mL and was statistically significantly higher (p < 0.001) than in healthy controls (88.89 [70.53; 107.75] ng/mL) (Fig. 1). Median anti-CarP was 110.81 [85.63; 150.54] in ACPA(+) RA, 128.34 [111.25; 165.08] in ACPA(–) RA, and 88.89 [71.37; 101.2] in the control; the differences were statistically significant (p < 0.001). The upper limit of the norm for anti-CarP was set at the 95th percentile of healthy control values and was 143.46 ng/mL. Among all patients with RA, 50 (33.3%) were anti-CarP-positive. In the ACPA(+) group, 22 (29.3%) patients were anti-CarP(+), in the ACPA(–) group, 28 (37.3%) patients were anti-CarP(+), and in the healthy control, one (2%) volunteer was anti-CarP(+).
When performing ROC analysis to assess the diagnostic significance of anti-CarP for the general population of patients with RA, the area under the curve was 0.783 ± 0.047 with a 95% CI of 0.691–0.874. This model was statistically significant (p < 0.001), with a cut-off point of 143.46 ng/mL; the specificity of the test was 96%, and the sensitivity was 36.7% (Fig. 2).
The predictive value of a positive result was 98%, the likelihood ratio of a positive result was 9.16, the test accuracy was 42.3%, and the Youden index was 0.33. The odds of having RA in the anti-CarP(+) patients were 12 times higher than in the anti-CarP(–)patients (95% CI of 1.58–91.58); the differences were statistically significant (p < 0.05).
When performing ROC analysis to assess the diagnostic significance of anti-CarP for RA among the ACPA(+) patients, the area under the curve was 0.706 ± 0.055 with a 95% CI of 0.598–0.813 (p = 0.002), with a cut-off point of 143.46 ng/mL, specificity 96%, sensitivity 32% (Fig. 3). This model was statistically significant (p < 0.001).
The predictive value of a positive result was 95.65%, the likelihood ratio of a positive result was 8, the test accuracy was 46%, and the Youden index was 0.28. The odds of having RA in the anti-CarP(+) patients were 9.96 times higher than in the anti-CarP(–) patients (95% CI of 1.27–78.26); the differences were statistically significant (p < 0.05).
When performing ROC analysis to assess the diagnostic significance of anti-CarP for RA among the ACPA(–) patients, the area under the curve was 0.860 ± 0.047 with a 95% CI of 0.768–0.951 (p < 0.001), with a cut-off point of 143.46 ng/mL, specificity 96%, and sensitivity 37.3% (Fig. 4). This model was statistically significant (p < 0.001).
The predictive value of a positive result was 96.55%, the likelihood ratio of a positive result was 9.33, the accuracy of the test was 52%, the Youden index was 0.33. The odds of having RA in the anti-CarP(+) patients were 14.3 times higher than in the anti-CarP(–) patients (95% CI of 1.83–111.55); the differences were statistically significant (p < 0.05) .
Then, the characteristics of anti-CarP(+) and anti-CarP(–) patients were assessed within the subtypes established by ACPA values. In the ACPA(+) RA group, the parameter “erosion count” in the anti-CarP(+) patients was statistically significantly higher (p = 0.044) than in the anti-CarP(–) patients and amounted to 12 [4; 26] and 3 [0; 16], respectively.
In the ACPA(–) RA group, the anti-CarP(+) patients showed a trend toward a lower total Sharpe score (56 [46; 96] versus 83 [55; 127] (p = 0.077)), probably mainly due to the narrowing of the interarticular spaces (55 [46; 82] versus 80 [55; 107] (p = 0.083)). A weak direct correlation between anti-CarP and DAS28soe was found (ρ = 0.239, p = 0.043).
For the ACPA(+) and ACPA(–) groups, no statistically significant differences were found in DAS28 values, CRP and ESR levels, as well as in the frequency of RF IgM seropositivity and in the extra-articular manifestations of the disease depending on the presence of anti-CarP.
DISCUSSION
According to our data, anti-CarP were detected in ACPA(+) and ACPA(–) patients with RA. In general, the diagnostic characteristics of anti-CarP for RA in our study are consistent with the data of other authors [7]. However, in our groups of patients, there were no statistically significant differences in the relative number of anti-CarP(+) patients depending on the ACPA subtype. This can probably be explained by the fact that the ACPA(–) patients were recruited taking into account their comparability with ACPA(+) in terms of age, duration of the disease, and therapy.
At the anti-Carb values selected by us as a cut-off point, they are inferior to ACPA in diagnostic sensitivity but have a comparable specificity. Due to this fact, they can serve as a promising auxiliary biomarker of RA to confirm the diagnosis, especially for the ACPA(–) subtype of the disease. The main difficulty in determining these autoantibodies is the lack of standard reference values and testing procedures. Studies typically use kits manufactured at research centers to perform specific research tasks, which may cause discrepancies in data and is a barrier to the introduction of anti-CarP in real clinical practice. It is also important to take into account that increased anti-CarP values were reported in patients with systemic lupus erythematosus (8.3–16.8%), Sjögren's disease (27–31%), and systemic scleroderma (5.8%) [23–26]. The detection of anti-CarP in other rheumatic diseases was associated with the presence of arthritis. This requires further research to clarify the specificity of these antibodies in the group of rheumatic diseases and analyze their prognostic and clinical significance.
Recruitment of patients taking into account the immunological subtypes of RA and subsequent determination of anti-CarP made it possible to assess the information brought by these autoantibodies to the already formed classification. For example, the ACPA(+) subtype is considered more “erosive” according to radiographic data in comparison with the ACPA(–) subtype, and in anti-CarP(+) patients, even within this group, a statistically significantly greater number of erosions was determined. Therefore, the combined seropositivity for ACPA, RF, and anti-CarP can be considered as a predictor of a more “aggressive” course of the disease and serve as a basis for early prescription of biologics.
In the group of ACPA(–) anti-CarP(+) patients, a correlation between anti-CarP and DAS28 was noted. Currently, RA is considered as a phenotypically heterogeneous syndrome, one of the components of the diagnosis of which is the determination of a limited range of autoantibodies (RF IgM and ACPA) and a set of relatively nonspecific clinical manifestations and laboratory abnormalities, reflecting the prevalence and severity of joint inflammation [27]. In the Swedish population-based EIRA study [28], among 554 ACPA- and RF-negative patients, in a more in-depth analysis, 43% proved to be seropositive when tests for additional ACPA, RF, and anti-CarP subtypes were combined. Thus, the use of anti-CarP analysis helps to delineate subtypes of the disease according to the immunological mechanisms of arthritis development and reduce the frequency of diagnosis of seronegative RA.
CONCLUSIONS
In this study, we investigated the predictive value of anti-CarP as an auxiliary biomarker in ACPA(+) and ACPA(–) subtypes of RA. In the ACPA(+) patients, anti-CarP helps to identify a more “erosive” subtype of the disease, and in the ACPA(–) patients it helps to reduce the relative number of seronegative ones. Further research is required to determine the optimal standards for laboratory diagnosis of anti-CarP and to clarify the diagnostic capabilities of these antibodies in the differential diagnosis of arthritis in other rheumatic diseases.
REFERENCES
Smolen, J.S., Aletaha, D., Barton, A., Burmester, G.R., Emery, P., Firestein, G.S., Kavanaugh, A., Mcinnes, I.B., Solomon, D.H., Strand, V., and Yamamoto, K., Rheumatoid Arthritis, Nat. Rev. Dis. Primers, 2018, vol. 4, no. 1, p. 18001. https://doi.org/10.1038/nrdp.2018.1
Dibrov, D.A., New laboratory biomarkers of rheumatoid arthritis, Nauchno-Prakt. Revmatol., 2021, vol. 59, no. 2, pp. 201–207. https://doi.org/10.47360/1995-4484-2021-201-207
Shi, J., Van Veelen, P.A., Mahler, M., Janssen, G.M.C., Drijfhout, J.W., Huizinga, T.W.J., Toes, R.E.M., and Trouw, L.A., Carbamylation and antibodies against carbamylated proteins in autoimmunity and other pathologies, Autoimmun. Rev., 2014, vol. 13, no. 3, pp. 225–230. https://doi.org/10.1016/j.autrev.2013.10.008
Wang, Z., Nicholls, S.J., Rodriguez, E.R., Kummu, O., Hörkkö, S., Barnard, J., Reynolds, W.F., Topol, E.J., Didonato, J.A., and Hazen, S.L., Protein carbamylation links inflammation, smoking, uremia and atherogenesis, Nat. Med., 2007, vol. 13, no. 10, pp. 1176–1184. https://doi.org/10.1038/nm1637
Mydel, P., Wang, Z., Brisslert, M., Hellvard, A., Dahlberg, L.E., Hazen, S.L., and Bokarewa, M., Carbamylation-dependent activation of T cells: a novel mechanism in the pathogenesis of autoimmune arthritis, J. Immunol., 2010, vol. 184, no. 12, pp. 6882–6890. https://doi.org/10.4049/jimmunol.1000075
O’Neil, L.J., Oliveira, Ch.B., Wang, X., Navarrete, M., Barrera-Vargas, A., Merayo-Chalico, J., Aljahdali, R., Aguirre-Aguilar, E., Carlucci, P., Kaplan, M.J., and Carmona-Rivera, C., Neutrophil extracellular trap-associated carbamylation and histones trigger osteoclast formation in rheumatoid arthritis, Ann. Rheum. Dis., 2023, vol. 82, no. 5, pp. 630–638. https://doi.org/10.1136/ard-2022-223568
Li, X., Wang, Zh., Yi, H., Xie, J., and Zhu, N., Diagnostic accuracy of anti-carbamylated protein antibodies in rheumatoid arthritis: a systematic review and meta-analysis, Clin. Lab., 2019, vol. 65, no. 12, pp. 1–5. https://doi.org/10.7754/clin.lab.2019.190419
Kuznetsova, P.A., Maslyanskiy, A.L., Lapin, S.V., Mazing, A.V., Bang, H., and Mazurov, V.I., Antibodies against post-translationally modified vimentin peptides in patients with rheumatoid arthritis, Sovrem. Revmatol., 2017, vol. 11, no. 3, pp. 44–49. https://doi.org/10.14412/1996-7012-2017-3-44-49
Brink, M., Verheul, M.K., Rönnelid, J., Berglin, E., Holmdahl, R., Toes, R., Klareskog, L., Trouw, L.A., and Rantapää-Dahlqvist, S., Anti-carbamylated protein antibodies in the pre-symptomatic phase of rheumatoid arthritis, their relationship with multiple anti-citrulline peptide antibodies and association with radiological damage, Arthritis Res. Ther., 2015, vol. 17, no. 1, p. 25. https://doi.org/10.1186/s13075-015-0536-2
Jiang, X., Trouw, L.A., Van Wesemael, T.J., Shi, J., Bengtsson, C., Källberg, H., Malmström, V., Israelsson, L., Hreggvidsdottir, H., Verduijn, W., Klareskog, L., Alfredsson, L., Huizinga, T.W.J., Toes, R.E.M., Lundberg, K., and Van Der Woude, D., Anti-CarP antibodies in two large cohorts of patients with rheumatoid arthritis and their relationship to genetic risk factors, cigarette smoking and other autoantibodies, Ann. Rheum. Dis., 2014, vol. 73, no. 10, pp. 1761–1768. https://doi.org/10.1136/annrheumdis-2013-205109
Ajeganova, S., Van Steenbergen, H.W., Verheul, M.K., Forslind, K., Hafström, I., Toes, R.E.M., Huizinga, T.W.J., Svensson, B., Trouw, L.A., and Van Der Helm-Van Mil, A.H.M., The association between anti-carbamylated protein (anti-CarP) antibodies and radiographic progression in early rheumatoid arthritis: a study exploring replication and the added value to ACPA and rheumatoid factor, Ann. Rheum. Dis., 2017, vol. 76, no. 1, pp. 112–118. https://doi.org/10.1136/annrheumdis-2015-208870
Truchetet, M., Dublanc, S., Barnetche, T., Vittecoq, O., Mariette, X., Richez, Ch., Blanco, P., Mahler, M., Contin-Bordes, C., and Schaeverbeke, T., Association of the presence of anti–carbamylated protein antibodies in early arthritis with a poorer clinical and radiologic outcome: data from the French ESPOIR cohort, Arthritis Rheumatol., 2017, vol. 69, no. 12, pp. 2292–2302. https://doi.org/10.1002/art.40237
Kolarz, B., Ciesla, M., Rosenthal, A.K., Dryglewska, M., and Majdan, M., The value of anti-CarP and anti-PAD4 as markers of rheumatoid arthritis in ACPA/RF negative rheumatoid arthritis patients, Ther. Adv. Musculoskeletal Dis., 2021, vol. 13, p. 1759720x2198986. https://doi.org/10.1177/1759720x21989868
Shi, J., van de Stadt, L.A., Levarht, E.W., Huizin-ga, T.W., Hamann, D., et al., Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis, Ann. Rheum. Dis., 2014, vol. 73, no. 4, pp. 780–783. https://doi.org/10.1136/annrheumdis-2014-eular.1192
Verheul, M.K., Böhringer, S., Van Delft, M.A.M., Jones, J.D., Rigby, W.F.C., Gan, R.W., Holers, V.M., Edison, J.D., Deane, K.D., Janssen, K.M.J., Westra, J., Brink, M., Rantapää-dahlqvist, S., Huizinga, T.W.J., Van Der Helm-van Mil, A.H.M., Van Der Woude, D., Toes, R.E.M., and Trouw, L.A., Triple positivity for anti-citrullinated protein autoantibodies, rheumatoid factor, and anti-carbamylated protein antibodies conferring high specificity for rheumatoid arthritis: implications for very early identification of at-risk individuals, Arthritis Rheumatol., 2018, vol. 70, no. 11, pp. 1721–1731. https://doi.org/10.1002/art.40562
Sidiras, P., Spruyt, D., Gangji, V., Imbault, V., Sokolova, T., Durez, P., Communi, D., Rasschaert, J., and Badot, V., Antibodies against carbamylated proteins: prevalence and associated disease characteristics in Belgian patients with rheumatoid arthritis or other rheumatic diseases, Scand. J. Rheumatol., 2021, vol. 50, no. 2, pp. 118–123. https://doi.org/10.1080/03009742.2020.1798500
Zhang, B., Lei, Yi., Li, X., Gao, Z., Xia, L., Lu, J., and Shen, H., Elevated levels of anti-carbamylated protein antibody in patients with rheumatoid arthritis: association with disease activity and bone destruction, J. Invest. Med., 2020, vol. 68, no. 6, pp. 1186–1192. https://doi.org/10.1136/jim-2019-001249
Elsayed, S.A., Esmail, M.A., Ali, R.M., and Mohafez, O.M., Diagnostic and prognostic value of anti-CarP antibodies in a sample of Egyptian rheumatoid arthritis patients, Clin. Rheumatol., 2019, vol. 38, no. 10, pp. 2683–2689. https://doi.org/10.1007/s10067-019-04616-z
Zhu, H., Zhao, L.J., Zhou, Y., and Chen, Y., Significance of anti-carbamylated protein antibodies in patients with rheumatoid arthritis-associated intersitial lung disease], Beijing Da Xue Xue Bao Yi Xue Ban, 2019, vol. 51, no. 6, pp. 1003–1007.
Castellanos-Moreira, R., Rodríguez-García, S.C., Gomara, M.J., Ruiz-Esquide, V., Cuervo, A., Casafont-Solé, I., Ramírez, J., Holgado, S., Gómez-Puerta, J.A., Cañete, J.D., Haro, I., and Sanmarti, R., Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: evidence of a new autoantibody linked to interstitial lung disease, Ann. Rheum. Dis., 2020, vol. 79, no. 5, pp. 587–594. https://doi.org/10.1136/annrheumdis-2019-216709
Vidal-Bralo, L., Perez-Pampin, E., Regueiro, C., Montes, A., Varela, R., Boveda, M.D., Gomez-Reino, J.J., and Gonzalez, A., Anti-carbamylated protein autoantibodies associated with mortality in Spanish rheumatoid arthritis patients, PLoS One, 2017, vol. 12, no. 7, p. e0180144. https://doi.org/10.1371/journal.pone.0180144
Prevoo, M.L.L., Van’t Hof, M.A., Kuper, H.H., Van Leeuwen, M.A., Van De Putte, L.B.A., and Van Riel, P.L.C.M., Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis, Arthritis Rheum., 1995, vol. 38, no. 1, pp. 44–48. https://doi.org/10.1002/art.1780380107
Ziegelasch, M., Van Delft, M.A.M., Wallin, P., Skogh, T., Magro-Checa, C., Steup-Beekman, G.M., Trouw, L.A., Kastbom, A., and Sjöwall, Ch., Antibodies against carbamylated proteins and cyclic citrullinated peptides in systemic lupus erythematosus: results from two well-defined European cohorts, Arthritis Res. Ther., 2016, vol. 18, no. 1, p. 289. https://doi.org/10.1186/s13075-016-1192-x
Nakabo, Sh., Yoshifuji, H., Hashimoto, M., Imura, Yo., Nakashima, R., Murakami, K., Kuramoto, N., Ito, Sh., Satoh, J., Tanaka, M., Fujii, T., Mimori, T., and Ohmura, K., Anti-carbamylated protein antibodies are detectable in various connective tissue diseases, J. Rheumatology, 2017, vol. 44, no. 9, pp. 1384–1388. https://doi.org/10.3899/jrheum.161432
Pecani, A., Alessandri, C., Spinelli, F.R., Priori, R., Riccieri, V., Di Franco, M., Ceccarelli, F., Colasanti, T., Pendolino, M., Mancini, R., Truglia, S., Barbati, C., Vomero, M., Sabatinelli, D., Morello, F., Valesini, G., and Conti, F., Prevalence, sensitivity and specificity of antibodies against carbamylated proteins in a monocentric cohort of patients with rheumatoid arthritis and other autoimmune rheumatic diseases, Arthritis Res. Ther., 2016, vol. 18, no. 1, p. 276. https://doi.org/10.1186/s13075-016-1205-9
Bergum, B., Koro, C., Delaleu, N., Solheim, M., Hellvard, A., Binder, V., Jonsson, R., Valim, V., Hammenfors, D.S., Jonsson, M.V., and Mydel, P., Antibodies against carbamylated proteins are present in primary Sjögren's syndrome and are associated with disease severity, Ann. Rheum. Dis., 2016, vol. 75, no. 8, pp. 1494–1500. https://doi.org/10.1136/annrheumdis-2015-207751
Nasonov, E.L., Avdeeva, A.S., and Dibrov, D.A., Rheumatoid arthritis as a clinical and immunological syndrome: focus on the seronegative subtype of the disease, Nauchno-Prakt. Revmatol., 2023, vol. 61, no. 3, pp. 276–291. https://doi.org/10.47360/1995-4484-2023-276-291
Reed, E., Hedström, A.K., Hansson, M., Mathsson-Alm, L., Brynedal, B., Saevarsdottir, S., Cornillet, M., Jakobsson, P.-J., Holmdahl, R., Skriner, K., Serre, G., Alfredsson, L., Rönnelid, J., and Lundberg, K., Presence of autoantibodies in “seronegative” rheumatoid arthritis associates with classical risk factors and high disease activity, Arthritis Res. Ther., 2020, vol. 22, no. 1, p. 170. https://doi.org/10.1186/s13075-020-02191-2
Funding
The article was prepared under the program no. 1021051402790-6 “Study of Immunopathology, Diagnosis and Therapy in the Early Stages of Systemic Rheumatic Diseases” (Nasonova Research Institute of Rheumatology)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Nasonova Research Institute of Rheumatology (protocol no. 22 dated December 2, 2021). Informed consent was obtained from all individual participants involved in the study.
Additional information
Translated by M. Batrukova
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dibrov, D.A., Avdeeva, A.S., Diatroptov, M.E. et al. Anti-Carbamylated Protein Antibodies in ACPA-Negative and ACPA-Positive Patients with Rheumatoid Arthritis. Dokl Biochem Biophys 517, 235–242 (2024). https://doi.org/10.1134/S1607672924700960
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1607672924700960