Abstract
Abutilon indicum Linn (A. indicum) is native to tropical and subtropical zones and traditionally used in ulcer, diabetes, piles, jaundice, gonorrhoea and leprosy. Erstwhile phytochemical analysis showed the presence of flavonoids, sesquiterpenes, gallic acid, β-sitosterols, geraniol, and caryophyllene. The study identifies the antidepressant potential of the crude methanolic extract of A. indicum (Ai.Cr). Crude methanolic extract of leaves and bark was prepared using maceration and freeze-drying. Forty Swiss-albino mice were divided into five groups containing eight mice each. Designated groups were administered with normal saline, Ai.Cr (30, 50, and 100 mg/kg) and diazepam (1 mg/kg) or fluoxetine (10 mg/kg) intra-peritoneally. Light and Dark Exploration (LDE), Elevated Plus Maze (EPM) and Hole Board (HB) test were used for anxiolytic activity testing, while forced swim and tail suspension model were used for the evaluation of antidepressant potential of Ai.Cr. Results showed that mice spent more time in light; passed more duration in open arms and raised number of head poking in respective anxiolytic LDE, EPM, and HB tests. Similarly, mobility time was raised in forced swim and tail suspension antidepressant testing. Ai.Cr has significant dose dependent antidepressant and anxiolytic potential, which peaks at highest dose (100 mg/kg) used in this study. A. indicum has significant pharmacological potential against anxiety and depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Abutilon indicum Linn (A. indicum) family (Malvaceae) locally known as Kangi booti and Peeli booti (called “Abutil” in Brazil) is a small shrub. Native to tropical and subtropical regions [1]. It is a medium sized, branched perennial shrub, going up to 2 m in height. Its leaves are alternate, cordate and acute with flowers of yellowish coloring comprising of 5 petals. Fruits have 15–20 chambers while seed color is blackish brown. Various parts of Abutilon indicum are used in traditional medicines. Abutilon indicum is used as a demulcent, aphrodisiac, laxative, diuretic, astringent, expectorant, tonic, anti-inflammatory, anthelmintic and analgesic agent [2]. It is also used therapeutically as a febrifuge, antiemetic, urinary, uterine discharges, piles, and lumbago [3]. Some precise studies have reported its seeds as effective decoction in the treatment of cough while its bark is valued as an anthelmintic and decreases perspiration. A wide-array of robust substances are reported in Abutilon indicum such as scopoletin and scoparone [4], saponins, flavonoids, alkaloids and essential oils, gallic acid, β-sitosterol, β-amyrin, eudesmol, eugenol, geraniol, and caryophylline [5], gossyptin-7-glucoside, cyanidin-3-rutinoside, sesquiterpene lactones (alantolactone and isoalantolactone, and gossypetin-8-glucoside [5].
Anxiety disorders are characterized by a state of excessive fear, actual threat, apprehension and uncomfortable feelings. As much as 13 to 28% of the American population is reported to be suffering from anxiety, at various stages of their lives and it is estimated to occur at the same ratio in other parts of world [6]. Generalized Anxiety Disorder is the most common form of anxiety disorder. Uncomfortable feeling of vague fear or apprehension can also be expressed the anxiety [6].
Recently there is increased research interest in anxiety disorders, because of a greater recognition of their burden and the implications associated with untreated illness. Clinical reviews have shown it is the risk factor for the development of other psychiatric disorders, substance abuse, cardiovascular diseases and gastrointestinal disorders. In clinical and population-based studies, the development of comorbidities makes the treatment difficult, and leads to low remission rates, poor prognosis and risk of suicide [7]. Anxiety has been associated with significant personal and societal ills, results in decreased work productivity, unemployment, and impaired social relationships [7].
Depression is clinically considered as a complex of different disorders. Each has its own definition, signs and symptoms, treatments and diagnostic criteria. Depression is characterized by depressed mood or loss of pleasure or interest in daily life activities. Except the depressed mood at least four further symptoms of depression are loss of interest, insomnia, change in appetite or weight, fatigue, suicidal thoughts and difficulty in concentration.
Etiology of depression is link to different types of neurotransmitters such as dopamine, serotonin and nor-adrenaline. Main cause of depression is imbalance between nor-adrenaline and serotonin in brain. Antidepressants produce therapeutic effect by inhibiting the reuptake of neurotransmitter that increases the amount of neurotransmitter at synaptic cleft [8].
Focus of pharmacological treatment is to restore the correct balance of nor-adrenaline and serotonin in brain. Clinically significant effects of all antidepressants start after about two weeks. Most common classes of antidepressants drugs are Selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs). Over the years, these classes have grown into a main stay in the treatment of anxiety and depression but progression of tolerance, greater chances of habituation, wide-scale drug abuses and manifestation of varied side effects remain a constant problem that scientific community long seeks to solve. The molecules with better tolerability and lesser abusive potentials are ideal candidates for optimized depression and anxiety treatments. The plant flora, hence, provides a suitable pool for such research.
The present study is aimed to screen A. indicum, the indigenous medicinal plant from Cholistan desert (Punjab, Pakistan) for anxiolytic and antidepressant potentials. This plant has showed potential to be a source of cheap and effective treatment and may open up new horizons in the therapeutics of anxiety and depression.
MATERIAL AND METHODS
Plant Material
Leaves and bark of Abutilon indicum were collected from the Cholistan desert in June 2019 in dried form. Dr. Nargis Naz, botanist from Department of Life Sciences, The Islamia University of Bahawalpur, Pakistan, helped in identification of plant. A voucher specimen was obtained and placed in the herbarium of Islamia University of Bahawalpur, Pakistan. The dried leaves and bark (aggregate weight: 5 kg) were crushed using mechanical grinders to obtain the fine powder that was subsequently immersed in 70% methanol for 24 h at room temperature. The solvent was decanted and then evaporated using rotary vacuum evaporator under reduced pressure to get the methanolic extract (90 g).
HPLC Analysis
The JASCO high-performance liquid chromatograph coupled with a UV–Vis multiwavelength detector (MD-910 JASCO) system was used for analytical HPLC. Evaluation of the analytical data were performed using a JASCO data processing system (DP-L910/ V). The separation was made at ambient temperature on a Waters Spherisorb 5 µm ODS2 4.6 × 250 mm column. Mobile phase used for separation consisted of water with 2% glacial acetic acid (solvent A) and water with 5% glacial acetic acid (solvent B). The flow rate was 0.5 mL/min, and the injection volume was 20 µL. The monitoring wavelength was 280 nm. The identification of each compound was based on a combination of retention time and spectral matching.
Mice
Male and female Swiss albino mice weighing 18–22 g were selected for experiments with each group containing six animals. Animals were housed under standard laboratory conditions of temperature (22 ± 2°C) and humidity (50–55%) along with 12 h light and dark cycle and animals were given standard diet and water ad libitum. Normal control group was administered with normal saline. Standard groups were administered with diazepam (1 mg/kg) and fluoxetine (10 mg/kg) for anxiety and depression respectively. Treatment groups were administered Ai.Cr in doses 30, 50, and 100 mg/kg intra-peritoneally. Elevated Plus Maze (EPM), Light and Dark Exploration, Hole Board behavioral models were used for the evaluation of anxiolytic activity whereas, forced swim and tail suspension model were used for evaluation of antidepressant potential of Ai.Cr.
Light/Dark Exploration Test
The apparatus had two boxes (25 × 25 × 25 cm3) separated by a wall. One was painted black and covered at the top (dark room), other box was painted white and open at the top (light room). The movement of each mice in light and dark box was observed i.e., time spent in each box for 5 min.
Elevated Plus Maze Test
EPM is widely used test to measure anxiety in rodents [14]. Apparatus have four arm- two open arms and two close arms. Animals of different treatment groups were treated and after 30 min, they were kept at the center of EPM apparatus facing towards closed arm. We recorded time spent (in s) in closed and open arm by each mouse.
Hole Board Test
Apparatus had a wooden chamber (40 × 40 × 25 cm3) having 16 holes of evenly spaced (diameter of each hole is 2.5 cm) from mice could peep through these holes. The number of head poking was recorded for 5 min.
Forced Swim Test
FST is most widely used in vivo model for determination of antidepressant activity. Apparatus made up of transparent plexiglass cylinder (20 × 12 cm). It was filled to 15 cm depth with water (24 ± 1°C). Pretest swimming session of 15 min was given to each animal, 24 h before final test session. For 5 min, the duration of immobility of each animal was noted. Mouse was immobile when no attempts of escape were made, only movement necessary to have its head out of water.
Tail Suspension Test
TST apparatus had a wooden chamber (70 cm high). Chamber consisted of a rod that was fitted between the wall of chamber, at height of 10 cm from top of the apparatus or 60 cm from the ground. Animals were hung by placing adhesive tape one inch from tip of tail with the rod. Each animal was given 15 min pretest session, 24 h before final test session. For 5 min of final test session, each animal was hung with rod and duration of immobility was noted. Mouse was immobile when no efforts was made to escape and passively hung with rod.
Statistical Analysis
The values are shown as mean ± SEM of 5 animals in each group. The results are evaluated by using one-way ANOVA and then compared with the of Normal Control group. The results are mainly considered significant (*) if p < 0.05, more significant (**) if p < 0.01, and highly significant (***) if p < 0.001.
RESULTS
Light and Dark Exploration Test
Average time spent by the mice in light and dark in different groups showed that Ai.Cr dose dependently raised the time spent in light (Fig. 1). Moreover, time spent by the mice in Ai.Cr treated group was significantly (p < 0.05) reduced during dark. Highest efficacy was observed in the mice treated with maximum dose of Ai.Cr.
Elevated Plus Maze Model
Elevated plus maze model showed relative improvement in time spent in open arm(s) after mice received various doses of Ai.Cr (Fig. 2). Similarly, time spent in close arm(s) was significantly (p < 0.05) reduced with the administration of increasing Ai.Cr doses.
Hole Board Model
Hole board test manifested significantly increased number of head poking by mice with the increasing doses administration of Ai.Cr (Fig. 3). The effects of Ai.Cr were compared with diazepam, which showed maximum number of head poking. The results are mainly considered significant (*) if p < 0.05, more significant (**) if p < 0.01, and highly significant (***) if p < 0.001.
Forced Swim Model
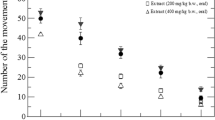
Mobility and immobility time of normal saline and Ai.Cr treated (at 30, 50, 100 mg/kg doses) groups showed a significant difference (Fig. 4). Ai.Cr treatment significantly (p < 0.05) raised the mobility time and reduced immobility time of mice in forced swim test. Ai.Cr 100 mg/kg almost equal the effects of fluoxetine.
Tail Suspension Model
Mobility and immobility time of normal saline, Ai.Cr treated (at 30, 50, 100 mg/kg doses) and fluoxetine treated groups are shown in Fig. 5. The comparison between both immobility and mobility time shows a dose dependent anti-depressant effect of Ai.Cr.
Phytochemical Testing
Phytochemical analysis using high performance liquid chromatography (HPLC) indicated that the Ai.Cr leaves and bark have carbohydrates and amino acid, non-drying oil possesses various fatty acids e.g., stearic, oleic, linoleic, palmitic etc. Phytochemical analysis of Ai.Cr showed that it contains saponins, alkaloids, terpenes, tannins, coumarins, glycosides, flavonoids and phenols (Table 1).
DISCUSSION
This study was designed to form the scientific basis of the indigenous medicinal plant Abutilon indicum for the treatment of psychiatric disorders like anxiety and depression. Anxiety and depression are the most common forms of psychiatric illnesses in modern day world, affecting almost 10–20% of the world population [9]. Major problems with the treatment of anxiety and depression are lack of definite diagnostic tests and dismissal of these disorders as mental illnesses. In the modern days, both these disorders have drawn significant attention of medical professionals and researchers, owing to their increasing prevalence [10].
Plant sources are the backbone of potential newer therapies. Abutilon indicum has been used since long in traditional medicine as anticonvulsant and antioxidant [11]. Plants having antioxidant properties and sedative potential can be useful for the treatment of epilepsy and anxiety [11]. Hence, this study hypothesized that Ai.Cr can be a valuable candidate in anxiety and depression.
Light and Dark Exploration, Elevated Plus Maze, and Hole Board are the most popular tests of currently available animal models of anxiety [12]. These behavioral test has a strong predictive validity for screening anxiolytic drugs [12]. Results displayed a dose dependent increase in the anxiolytic effect of the Ai.Cr. At higher doses, it is comparable to GABAergic effects at GABAA receptor. Reported mechanisms for anxiolytic activities of plant extracts are enhancement of GABA action at GABAA receptors, modulation of GABAA receptors and 5HT2A receptors antagonism [13]. Tail Suspension and Forced Swim Model results also indicated notable antidepressant potential of Ai.Cr. Both tests are valuable tool in drug discovery for high-throughput screening of prospective antidepressant compounds. Major antidepressant effects of plant extract includes the inhibition of monoamine oxidase-A, selective inhibition of serotonin and biogenic amine reuptake, or interaction of serotonin system [13, 14]. Fluoxetine, a major antidepressant drug, contain marked potential of antidepressant activity due to some selective serotonin reuptake inhibition.
Study proved that Ai.Cr has significant antidepressant and anxiolytic potential. These pharmacological effects are due to the presence of different chemical constituents. Flavonoids are the main constituent that is reported to possess the anxiolytic and antidepressant property. This study may serve as an important precedence for upcoming attempts to isolate the chemical constituents of this plant, which are responsible for these effects. Further, mechanistic studies are required to establish exact mechanism by which these chemical constituents exert their pharmacological effects in anxiety and depression.
CONCLUSIONS
It is plausible to conclude that Ai.Cr contains potential antidepressant and anxiolytic activities in relevant mice models, which serve as a precedent for future animal and human studies. Further molecular and pharmacokinetics studies can reveal the usefulness of plant in the anxiety and depression.
REFERENCES
Radhakrishnan, K., Mohan, A., Mohan, S.C., and Velavan, S., A comparison of chemical composition, antioxidant and antimicrobial studies of Abutilon indicum leaves and seeds, Res. J. Phytochem., 2017, vol. 11, pp. 11–19.
Rajurkar, R., Jain, R., Matake, N., Aswar, P., and Khadbadi, S., Anti-inflammatory action of Abutilon indicum (L.) sweet leaves by HRBC membrane stabilization, Res. J. Pharm. Technol., 2009, vol. 2, no. 2, pp. 415–416.
Gaind, K.N. and Chopra, K.S., Phytochemical investigation of Abutilon indicum, Planta Med., 1976, vol. 30, no. 2, pp. 174–188.
Rao, K., Chodisetti, B., Gandi, S., Giri, A., and Kishor, P.B., Regeneration-based quantification of coumarins (scopoletin and scoparone) in Abutilon indicum in vitro cultures, Appl. Biochem. Biotechnol., 2016, vol. 180, no. 4, pp. 766–779.
Sharma, P.V. and Ahmad, Z.A., Two sesquiterpene lactones from Abutilon indicum, Phytochemistry, 1989, vol. 28, no. 12, p. 3525.
Stein, D.J., Scott, K.M., de Jonge, P., and Kessler, R.C., Epidemiology of anxiety disorders: from surveys to nosology and back, Dialogues Clin. Neurosci., 2017, vol. 19, no. 2, p. 127.
Westwick, J.N., Hunter, K.M., and Haleta, L.L., Shaking in their digital boots: anxiety and competence in the online basic public speaking course, Basic Commun. Course Annu., 2015, vol. 27, no. 1, p. 10.
Sakalem, M.E., Seidenbecher, T., Zhang, M., Saffari, R., Kravchenko, M., Wördemann, S., and Ambrée, O., Environmental enrichment and physical exercise revert behavioral and electrophysiological impairments caused by reduced adult neurogenesis, Hippocampus, 2001, vol. 27, no. 1, pp. 36–51.
Okonogi, T., Nakayama, R., Sasaki, T., and Ikegaya, Y., Characterization of peripheral activity states and cortical local field potentials of mice in an elevated plus maze test, Front. Behav. Neurosci., 2018, vol. 12, p. 62.
Kessler, R.C., Chiu, W.T., Demler, O., and Walters, E.E., Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication, Arch. Gen. Psychiatry, 2005, vol. 62, no. 6, pp. 617–627.
Banerjee, P.N., Filippi, D., and Hauser, W.A., The descriptive epidemiology of epilepsy: a review, Epilepsy Res., 2009, vol. 85, no. 1, pp. 31–45.
Mahanthesh, M. and Jalalpure, S., Pharmacognostical evaluation and anticonvulsant activity of stem of Abutilon indicum Linn sweet, Int. J. Pharm. Pharm. Sci., 2016, vol. 8, pp. 58–70.
Perusini, J.N., Meyer, E.M., Long, V.A., Rau, V., Nocera, N., Avershal, J., and Fanselow, M.S., Induction and expression of fear sensitization caused by acute traumatic stress, Neuropsychopharmacology, 2016, vol. 41, no. 1, p. 45.
Zhang, Z-J., Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders, Life Sci., 2004, vol. 75, no. 14, pp. 1659–1699.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests. Statement on the welfare of animals. Animals Ethics Committee of animal handling of the University approved the protocols and study procedures.
Rights and permissions
About this article
Cite this article
Zhou, X., Hassan, W., Bakht, S. et al. Abutilon indicum Exhibits Anxiolytic and Antidepressant Effects in Mice Models. Dokl Biochem Biophys 500, 341–346 (2021). https://doi.org/10.1134/S1607672921050203
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1607672921050203