Abstract
The effect of γ-radiation on the level of nuclear DNA damage in onion seedlings (Allium-test) was studied using the comet assay. DNA breaks were first found in cells of onion seedlings exposed to low-dose radiation (≤ 0.1 Gy). Dose dependence of DNA damage parameters showed nonlinear behavior: a linear section in the low-dose region (below 0.1 Gy) and a dose-independent plateau in the dose range between 1 and 5 Gy. Thus, the comet assay can be used to estimate the biological effects of low-dose γ-radiation on Allium cepa seedlings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Artificial radionuclides in the environment are a source of irradiation of not only humans but also organisms of the biota. In most cases, living organisms are exposed to low-dose ionizing radiation. Aquatic organisms of the Yenisei River for a long time experience extra radiation load due to artificial radionuclides (including radioactive microparticles) as a result of long-term industrial discharges of the Rosatom Mining and Chemical Combine (MCC) into the river [1–3]. To simulate the impact of γ-radiation of radioactive particles, earlier we performed laboratory experiments with various plant and bacterial bioassays [4, 5], which showed their high sensitivity to low-dose γ-radiation [4, 5]. One of the bioassays was represented by the Allium-test [6], which showed a high level of chromosomal aberrations in seedling cells at low-dose irradiation.
Ionizing radiation induces DNA damage as a result of both direct energy action and indirectly (through the formation of free radicals). The result of physicochemical interaction between ionizing radiation and DNA are single-stranded and double-stranded DNA breaks, apurinic and apyrimidinic sites, base modifications, and cross-linking of DNA and proteins. These types of DNA damage can be detected using gel electrophoresis of individual nuclei (the comet assay) [7, 8]. The comet assay is commonly used to study animal and human cells [7, 8]. However, in several studies, the comet assay was used in plant systems to assess chemical toxicity [8, 9]. In some studies, the comet assay was used to assess radiation toxicity; however, in this case, high radiation doses were used (tens and hundreds Gy) [8, 10–13]. Only in one study [13], the genotoxicity of low-dose radiation (radioactive soils) was assessed using the Allium-test and the comet assay. However, the authors did not specify the irradiation doses of plants.
The purpose of this work was to evaluate the effect of γ-radiation (including small doses) on the level of DNA damage in onion seedling cells using the Allium-test.
Experiments on biotesting ionizing radiation were performed with seeds of onion (Allium cepa L.) cultivar Stuttgarter Risen. Seeds were preliminarily germinated in polypropylene containers on filter paper moistened with distilled water. For the experiment, sprouts 2–3 mm long were selected. Onion sprouts were irradiated with a γ-radiation source (137Cs with activity of 14 GBq) at the Budker Institute of Nuclear Physics (Novosibirsk) for 24 h. In total, we performed four experiments in 2016. In the experiments, the absorbed dose for onion seedlings was 0.02, 0.05, 0.1, 1, 3, and 5 Gy, which corresponded to a dose rate of 0.8, 2.1, 4.2, 42, 125, and 208 mGy/h. The γ-radiation dose rate values were determined by the distance between the source and the seedlings, were obtained by the calculation method based on the certificate rate of the exposure dose for the 137Cs source, and were verified by direct measurements with a DKS-AT1123 dosimeter (SPE Doza, Russia). The non-irradiated seedlings served as a negative control (dose rate in the control, 0.002 mGy/h), and the seedlings incubated in 0.5% H2O2 for 1.5 h served as a positive control. For irradiation, the seedlings were placed in transparent polypropylene containers on a bed of two layers of filter paper moistened in distilled water. For each irradiation level and control we used 15 seedlings. Experiments were performed at a temperature of 18–21°C without illumination.
DNA damage in seedling cells was assessed by the comet assay as described in [9]. Toot tips 0.3–0.5 mm long were cut off from the control and irradiated seedlings, placed in 300 μL of cold Tris-HCl buffer (400 mM, pH 7.5), and thoroughly minced. Then, 200 μL of the obtained suspension was added to 0.6 mL of low-melting agarose (1% low-melting agarose in sodium phosphate buffer) at 37°C, thoroughly mixed, and applied (200-μL aliquots) on pretreated slides (thin layer of 1% high-melting agarose in distilled water dried for 12 h), covered with coverslips, and incubated on ice for 3–4 min, after which the coverslips were removed. The preparations were placed in an electrophoresis chamber with a freshly prepared buffer (1 mM Na2EDTA and 300 mM NaOH, pH > 13) and incubated at 4°C for 15 min. Electrophoresis was also performed at 4°C for 30 min (40 V, 125 mA, 2 W). Then, the glasses were washed three times in neutralizing buffer (400 mM Tris-HCl, pH 7.5), stained with ethidium bromide (20 μg/mL, 100 μL per glass) for 15 min, and washed from excess dye in cold distilled water for 15 min.
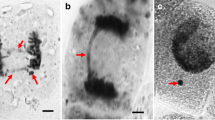
The results were analyzed with an AxioStar fluorescence microscope (Carl Zeiss) using the Zen software (Carl Zeiss). At each experimental point, no less than 100 comets were counted. Figure 1 shows the photographs of non-irradiated nuclei ((a), control) and nuclei damaged by radiation ((b), experiment, DNA comets) of onion cell nuclei. Data were processed using the CASPLab software (http://casplab.com/), and the results were presented as the following parameters:
(1) DNA in comet head, %;
(2) DNA in comet tail, %;
(3) comet tail moment (rel. units)—the product of the comet length from the middle of the head to the middle of the tail and the content of DNA in the comet tail.
The experimental data were analyzed by variation statistics methods using the STATISTICA 7.0 software package. The statistical significance of differences was assessed by Student’s t test.
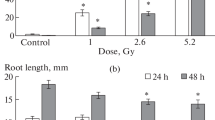
The methodology used [9] makes it possible to evaluate the total number of breaks in onion nuclear DNA (single-stranded and double-stranded DNA breaks). In all four experiments, we found that the accumulation of total DNA lesions in onion seedling nuclei is non-linear in the γ-radiation dose range used (from the background to 5 Gy). The data presented in Fig. 2a shows that the parameters of DNA damage (DNA in the comet tail and the comet tail moment) increased almost linearly with increasing irradiation dose and reach their maximum values at an irradiation dose of 0.02 Gy and higher. In the region of low irradiation doses (0.02–0.1 Gy), the parameters of DNA damage significantly differed from the parameters of the control (non-irradiated) plants.
At γ-irradiation doses above 1 Gy, no further increase in the total content of DNA lesions in seedling cell nuclei was observed; furthermore, the level of damage even slightly decreased (Fig. 2a). This reduction in DNA damage parameters for the comet tail moment (from 171 to 51 rel. units) was more pronounced than for the comet tail DNA (from 73 to 52%). It should be noted that, in the literature, the comet tail DNA is considered a more reliable parameter than the comet tail moment, because the changes in the comet tail width are not taken into account in the calculations of the comet tail moment [8]. The obtained maximum values of DNA damage parameters (comet tail DNA 73 ± 5% and comet tail moment 171 ± 21 rel. units) under γ-irradiation are close to the values of these parameters for the positive control, when the seedlings were incubated in 0.5% H2O2. For example, for the positive control, DNA damage parameters were as follows: comet tail DNA 80 ± 5% and comet tail moment 151 ± 17 rel. units).
Earlier, in experiments on γ-irradiation of onion seedlings in 2016–2017, we analyzed the cytogenetic indices (chromosomal aberrations and micronuclei) in the apical root cells at absorbed doses up to 10 Gy [6]. The data obtained showed that, as the irradiation dose increased to 5 Gy, the frequency of chromosomal aberrations (Fig. 2b) and the frequency of micronuclei (Fig. 2b) in onion seedling apical cells increased linearly. Under low-dose irradiation (≤ 0.1 Gy), the total frequency of chromosomal aberrations and micronucleus incidence was significantly higher than that in the control non-irradiated samples [6]. The detected fact of DNA damage in onion seedling cells at low doses (Fig. 2a) is additional evidence of the influence of low doses of γ-irradiation along with chromosomal aberrations and micronuclei.
However, the pattern of the dose curves for the parameters of DNA damage, as well as the frequency of micronuclei and chromosomal aberrations significantly differed under irradiation at doses higher than 0.02–1 Gy. For example, in the dose range from 1 to 5 Gy, the parameter of DNA damage (comet tail DNA) almost did not depend of the absorbed dose (i.e., there was a plateau in the dose dependence), whereas the frequency of micronuclei and chromosomal aberrations increased linearly (Fig. 2).
As mentioned above, the authors of [13] evaluated genotoxicity of radioactive soils using the Allium-test and the comets assay. They showed that, when the content of radium-226 in the soil increased 20 times (from 1 to 20 kBq/kg), the level of DNA damage in onion roots (comet tail moment) increased only twice. Although these authors did not specify the plant irradiation doses due to radioactive soils, it is obvious that the doses were low. The authors of [13] showed a linear dose dependence of the comet tail moment in onion roots on gamma-irradiation doses in the range from 5 to 50 Gy (dose rate, 1440 Gy/h). Differences in the pattern of dose dependences of DNA damage in our experiments (nonlinear dependence) and in the cited paper (linear dependence) can be explained by the nature and duration of irradiation. For example, in our experiments, irradiation of onion roots was chronic at a dose rate not more than 0.1–0.2 Gy/h, whereas irradiation in [13] was acute at a dose rate of 1440 Gy/h. In [10], experiments on gamma irradiation of Petunia leaves showed that, at low dose rates (0.33 Gy/min), the number of recorded DNA lesions was smaller than at high dose rates (5.15 Gy/min). It should be noted that the absorbed dose was the same (50 Gy) in both cases.
The authors of [12] also showed the nonlinear dose dependence of DNA damage during γ-irradiation of Arabidopsis roots in the absorbed dose range from 3 to 48 Gy and low dose rate of irradiation (0.1 Gy/h) in certain periods. The authors showed the saturation of the DNA damage dose curve starting from a dose of 3–6 Gy; in this case, the threshold level of comet tail DNA was 40%. Irradiation doses less than 3 Gy were not used, and DNA damage parameter values were not specified. Data obtained in [12] are consistent with the nonlinear dependence of DNA damage on the absorbed dose at chronic gamma-irradiation with a low dose rate, which was observed in our study (Fig. 2). Possibly, at a low dose rate, cells can effectively (but not necessarily correctly) repair occurring DNA breaks. The presence of unrepaired double-strand breaks in DNA may trigger cell death [14]. Probably, the level of DNA damage in cells, if there is time for DNA damage repair, is limited from top by a certain threshold, after which cells die. It is possible that such a threshold level of DNA damage is the maximum comet tail DNA value obtained by us (73 ± 5%, Fig. 2) or the maximum comet tail DNA value of 40% obtained in [12]. Conversely, chromosomal aberrations and micronuclei appear primarily as a result of improper repair of DNA double-strand breaks. Their accumulation in cells is not already detected by the comet assay, and the death of cells with such lesions requires passing one or several divisions. As a result, the frequency of chromosomal aberrations and micronuclei increases with increasing cumulative absorbed dose and may increase linearly, as in our case, in a certain dose range.
Thus, the DNA comet assay, which was used by us to assess DNA damage in onion seedling nuclei, showed an increase in DNA damage parameters (comet tail DNA and comet tail moment) in the γ-irradiation dose range from 0.02 to 5 Gy as compared to the control. It was shown for onion seedlings for the first time that, at low doses (≤0.1 Gy), DNA damage parameters significantly differed from the parameters in non-irradiated plants. The dose dependence of the DNA damage parameter (comet tail DNA) was nonlinear and included a linear segment at low doses (<0.1 Gy) and a dose-independent plateau in the range from 1 to 5 Gy. Earlier, a linear dependence of the frequency of micronuclei and chromosomal aberrations in onion cells was detected in this irradiation dose range. A possible explanation for the nonlinear effects of irradiation at doses of 1–5 Gy may be the existence of a threshold level of DNA damage, which is determined by the repair of breaks at low chronic irradiation dose rate. The fact of DNA damage in onion seedling cells at low doses is additional evidence (along with the chromosomal aberrations and micronuclei) of the effect of low-dose γ-irradiation on plants (Allium-test).
REFERENCES
Bolsunovskii, A.Ya. and Sukovatyi, A.G., Radiats. Biol. Radioekol., 2004, vol. 44, no. 3, pp. 393–398.
Bolsunovsky, A., Chem. Ecol., 2010, vol. 26, no. 6, pp. 401–409.
Bolsunovsky, A., Melgunov, M., Chuguevskii, A., Lind, O.C., and Salbu, B., Sci. Rep., 2017, no. 7, art. 11132, pp. 1–10.
Bolsunovsky, A., Frolova, T., Dementyev, D., and Sinitsyna, O., Ecotox. Environ. Saf., 2016, vol. 134, pp. 233–238.
Bolsunovsky, A.Ya., Dementyev, D.V, Trofimova, E.A., and Zotina, T.A., Dokl. Biol. Sci., 2017, vol. 475, pp. 157–160.
Bolsunovsky, A.Ya., Dementyev, D.V., Trofimova, E.A., Iniatkina, E.M., Kladko, Yu.V., and Petrichenkov, M.V., Dokl. Biochem. Biophys., 2018, vol. 481, pp. 181–185.
Olive, P.L., Int. J. Radiat. Biol., 1999, vol. 75, no. 4, pp. 395–405.
Collins, A.R., Mol. Biotechnol., 2004, vol. 26, pp. 249–261.
Gichner, T., Patková, Z., Száková, J., and Demnero-vá, K., Mutat. Res., 2004, vol. 559, pp. 49–57.
Dona, M., Ventura, L., Macovei, A., Confalonieri, M., Savio, M., Giovannini A., Carbonera, D., and Balestrazzi, A., J. Plant Physiol., 2013, vol. 170, pp. 780–787.
Koppen, G. and Cerda, H., LWT—Food Sci. Technol., 1997, vol. 30, pp. 452–457.
Tae Ho Ryua, Jin Kyu Kima, Jeong-Il Kimb, and Jin-Hong Kima, J. Environ. Radioact., 2018, vol. 181, pp. 94–101.
Saghirzadeh, M., Gharaati, M.R., Mohammadi, Sh., and Ghiassi-Nejad, M., J. Environ. Radioact., 2008, vol. 99, pp. 1698–1702.
Torudd, J., Protopopova, M., Sarimov, R., Nygren, J., Eriksson, S., Markova, E., Chovanec, M., Selivanova, G., and Belyaev, I.Y., Int. J. Radiat. Biol., 2005, vol. 81, pp. 125–138.
ACKNOWLEDGMENTS
We thank M. Petrichenkov from the Budker Institute of Nuclear Physics SB RAS for help with irradiation of onion seedlings.
Funding
Work on assessing DNA damage in onion seedling nuclei was performed using the equipment of the Core Facility for Microscopic Analysis of Biological Objects, Siberian Branch, Russian Academy of Sciences, funded under the research at the Institute of Cytology and Genetics, Siberian Branch, Russian Academy of Sciences. This work was supported in part by the Russian Foundation for Basic Research (project no. 18-44-240001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Bolsunovsky, A.Y., Dementyev, D.V., Frolova, T.S. et al. Effects of Gamma-Radiation on DNA Damage in Onion (Allium cepa L.) Seedlings. Dokl Biochem Biophys 489, 362–366 (2019). https://doi.org/10.1134/S1607672919060024
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1607672919060024