Abstract
Previously, we synthesized a dimeric dipeptide mimetic of the brain-derived neurotrophic factor (BDNF) loop 4, GSB-106, which, similarly to BDNF, activated TrkB, PI3K/AKT, and MAPK/ERK. When administered systemically, it exhibited neuroprotective, antidepressant, and antidiabetic activities and stimulated neurogenesis and synaptogenesis. In this study, we established that GSB-106 also exhibits the analgesic activity, typical for BDNF, which was revealed in rats in hot plate and tail flick tests 0.5–48 h after intraperitoneal injection at doses of 0.1 and 1 mg/kg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Brain-derived neurotrophic factor (BDNF) of the neurotrophin family regulates the development and maintenance of physiological functions of the central and peripheral nervous systems [1]. Its binding to the transmembrane tyrosine kinase receptor TrkB and activation of postreceptor signaling pathways PI3K/AKT and MAPK/ERK is associated with the manifestation of many biological effects including neuroprotective, antidepressant, antidiabetic, and analgesic ones [2]. For this reason, BDNF is considered as a pharmacologically promising compound for treating various neurological, psychiatric, endocrine, and other diseases. Attempts to use the full-length BDNF in replacement therapy were unsuccessful [3] due to its short half-life period and poor pharmacokinetic properties. In view of this, the creation of biologically stable low-molecular-weight BDNF mimetics is a relevant problem.
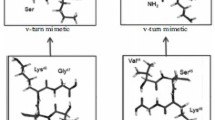
The mature BDNF is a 27-kDa protein composed of two identical polypeptide chains, each of which comprises seven beta-sheets connected via four hairpin loops, three of which (loops 1, 2, and 4) are exposed on the surface and may play an important role in the interaction with the receptor [4]. At the Zakusov Institute of Pharmacology, dimeric dipeptide mimetics of BDNF were designed and synthesized on the basis of beta-turns of its first (-D30-M31-S32-G33), second (-V44-S45-K46-G47), and fourth (-D93-S94-K95-K96) loops—bis-(N-monosuccinyl-L-methionyl-L-serine) heptamethylenediamide (working name GSB-214), bis-(N-hexanoyl-L-seryl-L-lysine) hexamethylenediamide (GTS-201), and bis-(N-monosuccinyl-L-seryl-L-lysine) hexamethylenediamide (GSB-106), respectively [5, 6]. In these compounds, the central dipeptide fragment of beta-turn, as a beta-turn that is most accessible to the receptor. The upstream residue is replaced with its bioisostere. The dimeric BDNF structure is reproduced in mimetics using hexamethylenediamine or heptamethylenediamine spacers (Fig. 1). All obtained mimetics activated the TrkB receptor; however, only GSB-106, mimetic of the fourth loop, which is most significantly exposed on the surface, activated the major signaling pathways of BDNF—AKT and ERK [6, 7]. This fact served as a basis for testing the hypothesis about the possibility of reproduction of the biological effects of BDNF by GSB-106. Indeed, in vitro and in vivo experiments showed that GSB-106 exhibited neuroprotective [7], antidepressant [8, 9], and antidiabetic [12] activity and stimulated neurogenesis and synaptogenesis [10, 11].
The aim of this work was to establish whether GSB-106 exhibits analgesic activity, which is characteristic of the full-length BDNF [13, 14].
The analgesic activity of GSB-106 was studied in the tail flick and hot plate tests in outbred male rats (200–220 g, Stolbovaya animal breeding facility) using analgesy meters (Ugo Basile, Italy) for analyzing acute somatic pain during thermal stimulation. The hot plate test was designed specifically for assessing the pain response by the behavioral components (paw licking and jumping), which are considered supraspinally integrated responses. The latent period of response in the tail flick test is determined by the spino-bulbo-spinal reflex, which is generated by motor neurons during stimulation of somatic and visceral afferent pathways [15]. We measured the latent period of the thermal exposure toleration by the animals at a temperature of 54 ± 0.1°C in the tail flick test and at 36 conventional units of an infrared heater in the hot plate test. Solutions of the tested compound were prepared in sterile distilled water ex tempore. The analgesic activity of GSB-106 was evaluated after its single intraperitoneal injection at doses of 0.1 and 1.0 mg/kg, which were selected on the basis of effective doses for antidepressant activity. The pharmacological effect of GSB-106 was recorded 0.5, 1, 24, 48, and 72 h after the peptide injection. The results are represented as a percentage of control. Each group included at least ten animals. Statistical analysis was performed using the descriptive statistics for the mean values with calculation of the standard error and the nonparametric Wilcoxon–Mann–Whitney U-test.
In the tail flick test, GSB-106 at a dose of 0.1 mg/kg significantly increased the pain threshold 0.5, 1, and 24 h after injection. This effect was not detected 48 h after injection. The maximum effect was observed 24 h after injection and constituted 149% of the effect in the control group. At a dose of 1.0 mg/kg, GSB-106 increased the pain threshold only 0.5 h after injection (Fig. 2).
Effect of GSB-106 on the pain threshold in the tail flick test in outbred rats. Here and in Fig. 3, data are represented as a percentage of control. GSB-106 doses (mg/kg) are indicated in the inset. * p < 0.05; *** p < 0.001 compared to the control.
In the hot plate test, an increase in the latent period of the response under the influence of GSB-106 was observed only at the maximum dose of 1.0 mg/kg. In a broader time range (0.5–48 h), activity reaching 145% (p < 0.001) of the activity in the control group of animals was detected 1 h after injection. The analgesic effect of GSB-106 was not observed 72 h after injection (Fig. 3).
According to the published data [14], under conditions of midbrain perfusion at a dose of 12 μg/day, BDNF exhibited analgesic activity in the tail flick test in rats starting from 4 h after injection. This effect was retained for 11 days with, being maximum 24 h after injection (134% of control), which is consistent with our results.
The quick response observed 0.5 and 1 h after GSB-106 injection may be due to the release of analgesic neuropeptides from stores under the influence of BDNF, whereas in the period of 24 h and later the mechanisms of synthesis and processing of neuropeptides are triggered. It was shown [14] that intracerebral injection of BDNF changes the level of pain modulators such as beta-endorphin, met-enkephalin, substance P, and neuropeptide Y.
Thus, GSB-106, a low-molecular-weight mimetic of the fourth loop of BDNF, which activates the AKT and ERK signaling pathways, similarly to the full-length protein, exerted a sustained analgetic effect. This allows considering the dipeptide GSB-106 as the basic structure for designing a new group of analgesic drugs.
ACKNOWLEDGMENTS
The study was performed in the framework of the State assignment for 2019–2021 “The Search for Pharmacological Tools for Selective Activation of Tyrosine Kinase Neurotrophin Receptor Signal Transduction Pathways As a Basis for Designing Drugs Without the Side Effects of Native Neurotrophins” (subject no. 0521-2019-0003).
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
REFERENCES
Kaplan, D.R. and Miller, F.D., Curr. Opin. Neurobiol., 2000, vol. 10, pp. 381–391.
Autry, A.E. and Monteggia, L.M., Pharmacol. Rev., 2012, vol. 64, no. 2, pp. 238–258.
Thoenen, H. and Sendtner, M., Nat. Neurosci., 2002, vol. 5, pp. 1046–1050.
Robinson, R.C., Radziejewski, C., Stuart, D.I., and Jones, E.Y., Biochemistry, 1995, vol. 34, no. 13, pp. 4139– 4146.
Gudasheva, T.A., Tarasyuk, A.V., Pomogaibo, S.V., Logvinov, I.O., Povarnina, P.Yu., Antipova, T.A., and Seredenin, S.B., Russ. J. Bioorg. Chem., 2012, vol. 38, no. 3, pp. 243–252.
Gudasheva, T.A., Tarasyuk, A.V., Sazonova, N.M., Povarnina, P.Yu., Antipova, T.A., and Seredenin, S.B., Dokl. Akad. Nauk, 2017, vol. 476, pp. 108–112.
Gudasheva, T.A., Povarnina, P.Yu., Logvinov, I.O., Antipova, T.A., and Seredenin, S.B., Drug Des. Devel. Ther., 2016, no. 10, pp. 3545–3553.
Seredenin, S.B., Voronina, T.A., Gudasheva, T.A., Garibova, T.L., Molodavkin, G.M., Litvinova, S.A., Elizarova, O.A., and Poseva, V.I., Acta Naturae, 2013, vol. 5, no. 4, pp. 116–120.
Tallerova, A.V., Povarnina, P.Yu., Blynskaya, E.V., Bueva, V.V., Gudasheva, T.A., and Seredenin, S.B., Khim.-Farm. Zh., 2018, vol. 52, no. 5, pp. 15–17.
Gudasheva, T.A., Povarnina, P.Yu., and Seredenin, S.B., Bull. Exp. Biol. Med., 2016, vol. 162, no. 9, pp. 448–451.
Gudasheva, P.Yu., Povarnina, T.A., and Antipova, S.B., Seredenin, Dokl. Biochem. Biophys., 2018, vol. 481, pp. 225–227.
Ostrovskaya, R.U., Yagubova, S.S., Gudasheva, T.A., and Seredenin, S.B., Bull. Exp. Biol. Med., 2017, vol. 164, no. 12, pp. 701–705.
Siuciak, J.A., Altar, C.A., Wiegand, S.J., and Lindsay, R.M., Brain Res., 1994, vol. 633, nos. 1–2, pp. 326–330.
Siuciak, J.A., Wong, V., Pearsall, D., Wiegand, S.J., and Lindsay, R.M., Eur. J. Neurosci., 1995, vol. 7, no. 4, pp. 663–670.
Le Bars, D., Gozariu, M., and Cadden, S.W., Pharmacol. Rev., 2001, vol. 53, no. 4, pp. 597–652.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Batrukova
Rights and permissions
About this article
Cite this article
Gudasheva, T.A., Konstantinopolsky, M.A., Tarasiuk, A.V. et al. Dipeptide Mimetic of the BDNF Loop 4 Possesses Analgetic Activity. Dokl Biochem Biophys 485, 123–125 (2019). https://doi.org/10.1134/S1607672919020121
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1607672919020121