Abstract
The modification of epoxy-amine systems with reactive monomeric derivatives based on guanidine has been attempted in order to create novel polymer coatings suppressing activity of pathogens. Preliminary chemical interaction of these compounds with the epoxy component allows their covalent incorporation into the epoxy-amine network to ensure prolonged action of the coating. The synthesized guanidine hydrosalicylate, hydro-4-aminosalicylate, hydro-5-sulfosalicylate, and dihydro-5-sulfosalicylate have been characterized by means of elemental and thermal analysis. The degree of hydrochloride substitution with the organic salt residue and temperature of onset of the thermal decomposition under argon as well as temperatures of the salts vitrification and melting have been determined. Solubility of the synthesized salts in a diane-epoxy oligomer has been estimated. It has been shown that the substitution of hydrochloride with the organic residue noticeably decreases the temperature of the onset of the reaction with the epoxy oligomer. Average functionality of the guanidine salts in the reaction with the epoxy oligomer has been determined; it has been revealed that most of the N‒Н groups of the modifiers are involved in the reaction, in certain cases these being the residues of the organic salts. Stoichiometry of the guanidine‒epoxy oligomer binary mixtures as well as this of the adducts synthesized with the oligomeric amine curing agent Jeffamine D-230 are presented. The initial tests of the obtained films have revealed pronounced bacteriostatic activity towards the methicillin-resistant S. Еpidermidis at the guanidine hydrosalicylate content as low 1 wt %, the parameter of the film forming suppression being 19.2%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Development and preparation of novel polymer protective coatings is among topical applied research issues [1–3]. The epoxy compositions are demanded materials of such type [4, 5]. They can form coatings due to the chemical processes resulting in the formation of three-dimensional polymer networks, exclusively resistant to negative environmental impact. In order to enhance the protective action of the coating, epoxy compositions can be modified with different compounds (see, for example, [6–8]), the modification with reactive compounds capable of be chemically involved into the three-dimensional polymer formation being especially advantageous. Such approach allows covalent incorporation of the modifier in the polymer network to ensure its prolonged action.
The limited resistance to biological damage is a serious issue of the coatings (including the epoxide ones) operating in contact with microorganisms, in particular, pathogens [9–12]. To improve the said parameter, various inorganic and organic additives have been used, among which reactive guanidine-containing modifiers are remarkable. The guanidines and their derivatives exhibit confirmed antimicrobial (antiviral, sporicidal, and fungicidal), insecticide, pesticide, algicide, cytotoxic, and anti-inflammatory activity, being simultaneously effective towards the aerobic and anaerobic microflora [13–18]. The water-soluble polymeric derivatives of guanidines have been widely used as the active component of many disinfecting formulations applied in agriculture and in medicine [19, 20]. However, guanidine-containing polymers are generally poor soluble in organic media (epoxy compositions), which does not allow their incorporation in the concentration sufficient to ensure pronounced biological activity. The studies on targeted modification of guanidine-containing oligomers via the synthesis of the salts with different organic acids [21–23] have demonstrated the increase in their solubility in the epoxy-amine systems, which has afforded uniform homogeneous coatings. However, it has been impossible to completely avoid the use of an organic solvent in the case of these systems. Another approach to enhance the modifiers solubility in an epoxy-amine system is to decrease its molecular mass, down to the monomeric guanidine. The monomeric guanidine bearing amino groups can itself chemically interact with the epoxy oligomers, whereas its derivatives (organic salts) can ensure additional biological action due to the presence of the organic acid residue. The effect of the nature of the acid residue on respiratory activity of model microorganisms has been revealed in [22].
The salts with salicylic acid and its derivatives have demonstrated the best biological effect among the guanidine-containing modifiers synthesized and studied earlier [21–25]. Therefore, this study aimed to prepare novel guanidine salts with salicylic acid and its derivatives for targeted covalent modification of the epoxy-amine coatings, which is an important step towards the development of efficient polymer coatings exhibiting biocide properties.
EXPERIMENTAL
The Epikote 828 diane epoxide oligomer (Hexion, USA) (I) and the Jeffamine D-230 oligooxypropylenediamine (Hunstman, USA) (II) as well as commercial (Merck) guanidine hydrochloride (CAS 113-00-8) (III) and hydrocarbonate (CAS 593-85-1) (IV) were used as the basic research objects. Other guanidine salts were prepared using the aromatic acids: salicylic, 4‑aminosalicylic (CAS 65-49-6; Aldrich), and 5-sulfosalicylic (CAS 97-05-2; Merck). Prior to the experiments, the diane epoxide oligomer was kept at 60°С during 3 h to remove the crystallites. Selected parameters of the epoxide oligomer and the curing agent are collected in Table 1.



Synthesis of the guanidine derivatives was performed from an aqueous-alcoholic solution of guanidine hydrocarbonate IV and equivalent amount of the corresponding organic acid; the evolved carbon dioxide and then the solvent were removed from the reaction mixture of a vacuum rotary evaporator. Complete removal of the solvents was ensured by trituration of the solid residue and additional drying in a vacuum oven at residual pressure not exceeding 10 Torr and temperature 60°С to constant mass. The components mass during the synthesis are given in Table 2.
Degree of substitution of hydrocarbonate with the organic acid residue was calculated from the data of elemental analysis performed using an EA 1112 instrument (Thermo Finnigan Italia S.P.A., Italy) via the following equation
with ntheor and npract being theoretical and experimental values of the molar content of an element.
IR spectra of the synthesized salts were measured in the ATR mode (ZnSe crystal) using a Nicolet 6700 spectrometer (Thermo Fisher Scientific, USA) over the 550–4000 cm–1 range (resolution 4 cm–1; averaging of 64 scans). To prepare the specimen, powder of a guanidine salt was put at the ZnSe crystal surface to completely cover it and pressed with a standard press.
Thermophysical properties of the guanidine salts, features of their interaction with the epoxy oligomer, and parameters of the cured systems were investigated by means of differential scanning calorimetry and thermal gravimetric analysis using DSC Q-100 and TGA Q-500 instruments (TA Instruments, USA) in the dynamic mode (constant heating rate 10 K/min). The experimental data were processed using TA Universal Analysis 2000 software package (V4.5A). The compositions were cured in an optimal regime elaborated basing on the TTT-diagrams for that epoxy-amine system [26, 27].
Biological activity of the cured epoxy-amine films was probed via the formation of biofilms at the polymer surface of the discs cut from the pristine and modified films. The discs were put in a Petri dish, the S. Еpidermidis 21555 (MRSA) inoculum was introduced in the titer of 106 CFU/mL, and the specimens were incubated during 3 days under stationary conditions at 36°С. Each 24 h, the plankton was removed, gently washed three times with a physiological solution, and transferred in new Petri dishes with fresh medium.
Analysis of density (biomass) of the biofilms (CV-test) was performed via coloring with a 0.1% solution of gentian violet. The discs were washed in a physiological solution to remove not attached cells, transferred into test tubes, and dried at 50°С; then the dye was introduced to completely cover the discs. Upon incubation during 15 min at room temperature (25°С), the dye was removed, and the discs were washed until the washings were colorless. The discs were dried, and the dye was eluted with 70% alcohol during 15–20 min. The colored alcoholic solution was transferred to a for immunological tests (100 μL per a well). The absorbance of the alcoholic solution was measured at wavelength 580 nm using a Bioscreen plate reader equipped with an automated system (Labsystems, Finland) and the original software. The effect of the organic guanidine salt was expressed as the fraction of the inhibition of the film formation with respect to the reference:
RESULTS AND DISCUSSION
Four salts were synthesized from the commercial guanidine hydrocarbonate; however, the degree of substitution of hydrocarbonate with the organic acid could not be estimated by means of NMR (as, for example, in the case of oligohexamethyleneguanidine [22, 23]), since the guanidine–acid residue could not be correctly determined from the 1Н NMR spectra due to exchange processes involving the guanidine protons and residual protons of the solvent (DMSO-d6 or D2O). In the case of the 13С NMR spectra, the experiment conditions affording adequate ratio of the integral intensities could not be set up even in the Inverse Gate mode. Therefore, the elemental analysis method was used. The ratios between the principal elements: carbon, hydrogen, nitrogen, and sulfur (in the case of sulfosalicylates) were determined (Table 3). The obtained data were compared to the values calculated from the theoretical formulas of the salts; they turned out to be very close, the deviation not exceeding 6%. Hence, it could be argued that the salts of guanidine and the organic acids with high degree of substitution were obtained.
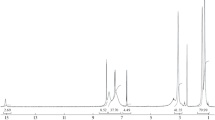
The obtained salts were investigated by means of IR spectroscopy. The IR-Fourier spectra of the salts are displayed in Fig. 1. The spectral bands were assigned using the reference data [28–31]; the results are collected in Table 4. It was found that the measured IR spectra contained the absorption bands characteristic of the expected functional groups of the synthesized salts: hydroxylic, amine, carboxylic, sulfonic, and aromatic ones. It is important to note that the spectra of the compounds contained strong absorption bands of the νas and νs vibrations of the –СОО‒ groups. The νas and νs bands of the –\({\text{SO}}_{3}^{ - }\) groups were observed in the spectra of the hydro-5-sulfosalicylate and dihydro-5-sulfosalicylate. At the same time, characteristic bands of vibrations of non-ionized carboxylic groups were absent in the spectra. The presence of the guanidinium I and II bands in the spectra of the synthesized compounds also confirmed their salt nature. Hence, the results of the spectral investigation led to the conclusion that the synthesized products were the salts of guanidine and salicylic acid (or its derivatives).
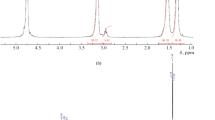
The guanidine salts were investigated by means of thermal analysis. Figure 2 displays the TGA thermograms used to estimate the temperature of the specimens decomposition and the DSC thermograms revealing that the synthesized salts exhibited the crystallization and vitrification transitions, i.e. were semicrystalline, in contrast to completely crystalline guanidine hydrochloride. In other words, the presence of a bulky organic acid residue hindered the guanidine salts crystallization.
Temperatures of the corresponding transitions are shown in Table 5. From the table it is to be seen that the melting temperature of the crystalline part of the organic salts was noticeably lower in comparison with guanidine hydrochloride and hydrocarbonate and, similarly to the vitrification temperature, was strongly changed depending on the salt nature. Evidently, the presence of polar sulfonic or amino groups led to stronger intermolecular interactions, which caused higher temperature of the phase transitions. Temperature of the decomposition onset (140 to 250°С) limited the temperature regime of further covalent incorporation of the synthesized salts in the epoxy-amine systems. Moreover, from the data in Table 5 it could be concluded that guanidine hydrosalicylate, being transformed in the liquid state at lower temperature affording the chemical interaction and revealing sufficient thermal stability, was the most interesting for further investigation.
The synthesized and characterized guanidine salts were introduced in the epoxy-amine mixtures. Preliminary investigation of solubility of those salts in the epoxy-amine systems revealed that up to 10 wt % of the guanidine salts introduced in the epoxy oligomer as well as the curing agent media was nor precipitated. The most rational way to introduce the salt used in this study was its dissolution in the amine, followed by the addition of stoichiometric amount of the epoxide oligomer. It should be noted that the stoichiometry of the system should be calculated accounting for the epoxy groups reaction with the guanidine protons as well as the functional groups of the organic acid residue in the salt. Possibility of the latter process was estimated by means of DSC (Fig. 3).
It was revealed that noticeable chemical processes in the absence of a catalyst were observed only in the cases of sulfosalicylic and (less pronounced) salicylic acids. However, those processes occurred at temperature exceeding 120–150°С (as expected for aromatic nucleophiles) and could not interfere the stoichiometry of the curing at lower temperature (according to the TTT-diagrams, the curing was performed up to 100°С).
Further, the exothermic effect of the chemical interaction between one of the guanidine salts (hydrosalicylate) and the epoxide oligomer was estimated. To do so, the DSC thermograms were recorded at different molar ratios between the epoxy group and the reactive groups of the modifier (Fig. 4). It is known that, other conditions being the same, the system stoichiometry affects the heat effect of the reaction, which is the highest at the equivalent ratio of the components [32]. Concentration dependence of the heat effect calculated from the thermograms in Fig. 4, revealed that the strongest heat evolution was reached at the average functionality of the hydrosalicylate equal to six (Fig. 5), likely corresponding to five protons of guanidine and a carboxylic group of salicylic acid, the latter process being probably catalyzed by the amino group of guanidine. In the cases of the hydrosulfosalicylates and aminosalicylate the experiments were not necessary: the additional functionality of the sulfonic group and two protons of the aromatic amine should be evidently accounted for.
The actual stoichiometric ratios for different salts with the epoxide oligomer and the epoxy-amine system are shown in Table 6. Those values afforded completely cured homogeneous transparent films for further experiments.
It is important to note that the model salts synthesized and characterized in this study allowed elucidation of the involvement of different fragments of the reactive guanidine modifiers of different molecular mass in the epoxide oligomer curing, but can also be used as independent modifiers covalently incorporated into the epoxy-amine network.
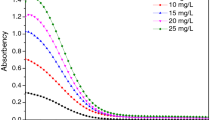
Biological activity of the obtained epoxy-amine films modified with different amounts of guanidine salicylate with respect to the biofilms of test microorganisms was assessed. In the preliminary experiments, the films revealed pronounced bacteriostatic activity towards the epidermal staphylococcus S. Еpidermidis 21555 (MRSE).
Staphylococcus Еpidermidis is a major commensal of human skin, however, it can cause infections related to medical treatment. S. Еpidermidis has been recognized among the most widespread causes of the hospital infections, the infecting frequency being as high as in the case of S. Аureus. Due to the formation of biofilms, S. Еpidermidis, especially the methicillin-resistant strains, exhibit high epidemic potential, causing heavy and difficult to treat infections [33]. The experiments revealed (Fig. 6) that at the guanidine hydrosalicylate content in the coating of 1 wt %, the parameter of the biofilm forming inhibition equaled 19.2% with respect to the reference sample (without a modifier), being 43.2% at 5 wt% of the additive.
CONCLUSION
In this study, the epoxy-amine systems based on the diane epoxide oligomer and the oligomer amine curing agent were modified with reactive monomeric derivatives of guanidine. The possibility of covalent binding of the modifier with the forming network was demonstrated, which should allow prolonged bacteriostatic effect of the coating. The salts of guanidine with organic acids were synthesized, and their solubility in the diane epoxide oligomer was estimated. It was found that the exchange of hydrochloride with the organic residue noticeably decreased the temperature of the onset of the reaction with the epoxy oligomer. Furthermore, average functionality of the guanidine salts with the epoxy oligomer was determined, and it was shown that most of the N‒Н groups of the modifiers were involved in the reaction. For example, the organic acid residue took part in the chemical interaction. Stoichiometry of the binary guanidine–epoxide oligomer systems as well as that of the obtained adducts with the oligomer amine curing agent were determined. It was marked that up to 10 wt % of the salt could be incorporated using the suggested approach, the most rational procedure being dissolution of the guanidine salt in the amine, followed by addition of the stoichiometric amount of the epoxide oligomer. Preliminary tests of the obtained films revealed pronounced bacteriostatic activity towards the methicillin-resistant strain of S. Еpidermidis.
In summary, targeted modification of the guanidine-containing compounds and optimization of the procedures to incorporate them in the epoxy-amine compositions as well as formation of the final coatings affords novel materials with pronounced antimicrobial action, safe to human and animals. Such materials are demanded at medical, pharmaceutical, and food facilities.
REFERENCES
G. De With, Polymer Coatings: a Guide to Chemistry, Characterization, and Selected Applications (Wiley-VCH, Weinheim, 2018).
Polymer Coatings: Technologies and Applications, Ed. by S. M. Rangappa, J. Parameswaranpillai, and S. Siengchin (CRC Press, Boca Raton, 2020).
F. N. Jones, M. E. Nichols, and S. P. Pappas, Organic Coatings: Science and Technology (Wiley, London, 2017).
E. Petrie, Epoxy Adhesive Formulations (McGraw-Hill Education, New York, 2005).
Resins for Surface Coatings, Ed. by P. K. T. Oldring (Wiley, London, 2001).
K. Y. Cao, Z. X. Yu, L. J. Zhu, D. Yin, L. G. Chen, Y. Jiang, and J. Wang, Surf. Coat. Technol. 465, 771 (2021).
S. Kopsidas, G. B. Olowojoba, A. J. Kinloch, and A. C. Taylor, Int. J. Adhes. Adhes. 104, 1 (2021).
A. M. Aguirre-Guerrero and R. M. de Gutierrez, Construct. Build. Mater. 268, 1 (2021).
A. V. Yastrebinskaya, L. Yu. Matveeva, and A. S. Edamenko, Diffus. Defect Data, Pt. B, 299, 55 (2020).
M. Gavrilov, V. Erofeev, and V. Afonin, AlfaBuild 20, Art. No. 2006 (2021).
S. Taioli, P. E. Trevisanutto, P. Vera, S. Simonucci, I. Abril, R. Garcia-Molina, and M. Dapor, J. Phys. Chem. Lett. 12, 487 (2021).
J. Zhang, B. Zhu, H. Wang, C. Zhang, W. Zeng, and Q. Zhou, Front. Mater. 8, 730627 (2021).
M. Kh. Rauf, Imtiaz-Ud-Din, and A. Badshah, Expert Opin. Drug Discovery, No. 8, 1 (2013).
I. S. Ryzhkina, L. I. Murtazina, E. D. Sherman, M. E. Pantyukova, E. M. Masagutova, T. P. Pavlova, S. V. Fridland, and A. I. Konovalov, Dokl. Ross. Akad. Nauk 438 (2), 207 (2011).
M. R. Menyashev, A. I. Martynenko, N. I. Popova, N. A. Klescheva, and N. A. Sivov, Polym. Sci. Ser. B 58 (5), 394 (2016).
Yu. I. Musaev, E. B. Musaeva, and I. Kh. Kirzhinova, Fund. Issledovaniya, No. 12, 139 (2011).
E. A. Borodina, N. A. Orlova, Yu. V. Gatilov, and O. I. Sal’nikova, Zh. Org. Khim. 51, 1778 (2015).
M. G. Voronkov, L. I. Belousova, Yu. N. Pozhidaev, and N. N. Vlasova, Zh. Obshch. Khim. 73, 1311 (2003).
I. I. Vointseva and P. A. Gembitskii, Polyguanidines – Disinfectants and Polyfunctional Additives to Composite Materials, (LKM-Press, Moscow, 2009) [in Russian].
S. Yu. Hashirova, Yu. I. Musaev, A. K. Mikitaev, Yu. A. Malkanduev, and M. H. Ligidov, Poly, Sci. Ser. B 51 (9), 1723 (2009).
I. N. Senchikhin, E. S. Zhavoronok, A. V. Matveev, O. Ya. Uryupina, and V. I. Roldugin, Kolloid. Zhurn. 80, 324 (2018).
E. S. Zhavoronok, I. P. Sedishev, A. V. Safonov, and I. N. Senchihin, Polym. Sci., Ser. A 61 (5), 610 (2019).
E. S. Zhavoronok, I. P. Sedishev, M. S. Merkulova, O. Ya. Uryupina, and I. N. Senchihin, Polym. Sci., Ser. B 63 (1), 31 (2021).
I. N. Senchikhin, E. S. Zhavoronok, E. V. Kharitonova, and V. I. Roldugin, Tonkie Khim. Tekhnologii 11 (6), 98 (2016).
I. N. Senchikhin, E. S. Zhavoronok, A. V. Matveev, O. Ya. Uryupina, V. I. Roldugin, Inzhenernyi Zhurnal: Nauka I Innovatsii 72 (12), 1 (2017).
E. S. Zhavoronok, I. N. Senchikhin, I. E. Pchelintsev, and V. I. Roldughin, Polym. Sci., Ser. B 60 (2), 188 (2018).
E. S. Zhavoronok and I. N. Senchikhin, J. Appl. Polym. Sci. 137 (44), 1 (2020).
A. Smith, Applied Infrared Spectroscopy: Fundamentals Techniques and Analytical Problem-Solving (Wiley-Interscience; New York, Chichester, Brisbane, Toronto (1979).
R. M. Silverstein, F. X. Webster, and D. J. Kiemle, Spectrometric Identification of Organic Compounds (Willey, Rio de Janeiro, 2005).
L. Bellamy, The Infra-Red Spectra of Complex Molecules (Springer, New York, 1975).
K. Nakanishi and P. A. Solomon, Infrared Absorption Spectroscopy (Holden Day, San Francisco, 1977).
F. G. Garcia, P. M. Silva, B. G. Soares, and J. R. Briones, Polym. Test. 26, 95 (2007).
E. Lee and F. Anjum, StatPearls [Internet], Staphylococcus Epidermidis. www.ncbi.nlm.nih.gov/books/ NBK563240. Accessed April 24, 2023.
ACKNOWLEDGMENTS
The experiments were performed using the equipment of the Centers for Collective Usage of Frumkin Institute of Physical Chemistry and Electrochemistry, RAS and of Russian Technological University MIREA (Agreement no. 075-15-2021-689, 01.09.2021).
Funding
This study was financially supported by the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Karpushkin
Rights and permissions
About this article
Cite this article
Senchikhin, I.N., Merkulova, M.S., Sedishev, I.P. et al. Epoxy-Amine Systems with Reactive Guanidine Derivatives. Polym. Sci. Ser. B 65, 133–143 (2023). https://doi.org/10.1134/S1560090423700896
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090423700896