Abstract
To prepare a uniformly network structured stimulus-responsive copolymer, organic polyethylene imine (PEI), polyethylene glycol glycidyl ether (PEGO) and inorganic calcium phosphate oligomers (CPO) as precursors, were used to prepare the PEI-PEGO-CPO hydrogels. The structure and properties of the products were characterized using infrared, 1H NMR and swelling behavior tests. The influence of CPO on temperature and pH sensitivity of the hydrogels was systematically investigated. The hydrogels were switched at temperatures between 20 and 60°C, pH values between 8 and 13. The results showed that the addition of CPO can effectively improve the temperature and pH responsive properties of the hydrogels. In addition, the hydrogels showed excellent reversibility for swelling–deswelling characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Isaka [1] found that the swelling of polyacrylamide gel was completely opaque to the reversible change of transparency with the change of temperature in 1970s, and the concept of intelligent hydrogel was first proposed. Environmental responsive hydrogels are often needed rapid response characteristics in chemical sensors, chemical microvalves, artificial muscles, drug controlled release carriers, and material separation. Improving the response rate of intelligent hydrogels has become one of the important topics in the field of intelligent hydrogel research [2]. Changing the hydrogel network to prepare porous structure is one of the most important ways to improve the response rate of intelligent hydrogel [3].
Polymerization (or copolymerization) of bi-functional (or multi-functional) monomers and crosslinking of linear pre-polymers using chemical reactions are widely used to synthesize polymer network in chemical gels. We have developed several types of gels synthesized by addition reactions between multi-functional monomers as “joint unit” sources and α,ω‑bifunctional monomers as “linker unit” sources in some solvents, based on the joint and linker concept. The gels formed a homogeneous network structure with extremely narrow mesh size distribution, and their mesh size could be controlled by the molecular length of the bi-functional monomers [4–6]. However, those gels were too fragile to evaluate their mechanical properties.
The addition of inorganic substances can enhance the strength of hydrogels. Organic-inorganic composites, especially polymer-inorganic hybrid materials, integrate both superiorities of organic phases for flexibility and inorganic phases for strength [7, 8]. However, traditional inorganic composite hydrogels show some obvious drawbacks, especially low swelling rate, resulting in limitation for their widespread applications. It is mainly due to the fact that inorganic particles are trapped in the structure of hydrogel networks, which impede the migration of water molecules. The research on organic and inorganic copolymerization was suggested by Ruikang Tang [9]. It showed that new organic-inorganic composites can be prepared by copolymerization. Its unique advantage is to produce homogenous organic-inorganic composites without interphase boundary. As a result, copolymerization can promote more composite materials with optimized performances due to their structural uniformity and continuity. The research provides a new insight for preparing inorganic-organic gel at molecular level.
In this study, calcium phosphate oligomers (CPO) are used as the inorganic precursors, which can be crosslinked with polyethyleneimine and poly(ethylene glycol) diglycidyl ether to form a homogeneously structured organo-inorganic hybrid gel without interphase boundary. As the expectation, this copolymer exhibits a prominent improvement of equilibrium swelling rates.
EXPERIMENTAL
Materials
Polyethyleneimine: Sigma-Aldrich, AR, Mn ≈ 1.8 × 103. PEG (Mn = 103) and sodium hydroxide were purchased from Tianjin Damao chemical reagent Co, China. Tetrabutylammonium bromide (Bu4NBr): Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Epichlorohydrin (ECH): Tianjin Yongda chemical reagent Co., Ltd (Tianjin, China), AR. Calcium chloride dihydrate (CaCl2⋅2H2O) and ammonium persulfate (APS) were purchased from Aladdin’s Reagent Co., China. Ethanol and phosphoric acid (≥85%) triethylamine (TEA) were purchased from Macklin and used as received. Calcium phosphate oligomers (CPO) [9] used as the inorganic precursors and PEGO were synthesized as described in the literature [10]. All other chemicals and solvents were of analytical grade.
Characterization
The samples were characterized by Fourier transform infrared spectroscopy with potassium bromide pellets (Billerica, Massachusetts, USA, in the range of 4000~450 cm–1).
The interaction between CPO and PEI-PEO chains was determined by 400 MHz solid state NMR spectrometer (VNMRS-400, Agilent, America).
UV–Vis spectra were recorded on a TU-1901 ultraviolet–visible spectrophotometer with distilled water used as the solvent.
Viscoelasticity Measurements of PEGO-PEI and PEGO-PEI-CPO were conducted on a Discovery HR-1 rheometer (USA) with a DG41Ti rotor. The shear rate was 6 s−1, and the temperature was ~40–60°C, attained at a heating rate of 1.0 deg/min. The test system was a binocular tube.
Synthesis of Calcium Phosphate Oligomers (CPO)
Calcium phosphate oligomers (CPO) [9] used as the inorganic precursors was synthesized as described in the literature (Scheme 1). In brief, 4.704 g CaCl2⋅2H2O is dissolved in 1.60 L of ethanol to form a clear alcoholic solution. 88.716 mL TEA is added to the above solution under magnetic stirring for 30 min at room temperature. The H3PO4 alcoholic solution (1.672 mL H3PO4 dissolved in 80 mL of ethanol) is then added dropwise under magnetic stirring for 12 h at room temperature. The CPO gel is obtained by centrifugation at 8000 rpm, washed with ethanol several times to remove residual TEA. The CPO is re-dispersed in ethanol to form a homogeneous emulsion with a concentration of about 20 mg/mL for future use.

Scheme 1.
Synthesis of Polyethylene Glycol Glycidyl Ether (PEGO)
PEGO [10] was synthesized as described in the literature (Scheme 2). PEGO was synthesized via the reaction of PEG and ECH in the presence of NaOH. The synthesis can be briefly described as follows: PEG (50 g) was added into a three-neck flask equipped with a mechanical stirring rod and condenser with stirring at 45°C until the PEG was fully dissolved. Bu4NBr (0.6447 g) and ECH (31.6 mL) were added to the PEG solution. The above mixture was mechanically stirred for 15 min at 55°C. NaOH (8 g) was added to the mixture and stirred for 4 h. The ethanol solution of sodium hydroxide (8 g NaOH + 72 mL ethanol) was added, and the reaction was continued for 2 h. The generated sodium chloride was removed via hot filtration. The unreacted ECH and ethanol were removed via vacuum distillation. The product was obtained in 90.1% yield.

Scheme 2.
Preparation of the Hydrogels
The synthesis of hydrogels was briefly described as follows: PEI (2.79 g, 1.55 mmol), PEGO (8.62 g, 7.75 mmol) and CPO (20 mg/mL, 5 mL) were added to distilled water (37.74 g) and sonicated to obtain a homogeneous aqueous solution. The resulting system was stirred for 30 min to ensure it was completely mixed and the reaction was allowed to proceed without agitation for 24 h at 45°C. The product was purified in a large amount of distilled water for at least one week in order to wash out the low molecular weight components in the system. The reaction is outlined in Scheme 3.

Scheme 3.
RESULTS AND DISCUSSION
Infrared Spectroscopy Analysis
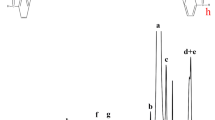
Figure 1 shows the FTIR spectra recorded for PEI (curve 1), PEGO (curve 2), PEI-PEGO (curve 3), CPO (curve 4), and hydrogel PEI-PEGO-CPO (curve 5).
The intensity of the absorption peak at about 1024 cm–1 (Fig. 1, curve 1) derived from the tertiary amine group, indicating that the compound is a branched polyethyleneimine. The intensity of the characteristic absorption peaks at about 1279, 946, 843 cm–1 (Fig. 1, curve 2) are the ternary ring epoxide, the weak absorption peaks at 3200–3600 cm–1 indicate that a small amount of –OH in the synthesized poly(ethylene glycol) glycidyl ether has not been completely epoxidized.
Comparing with curve 1 and curve 2 (Fig. 1), C–N stretching vibration peak of tertiary amine at 1048 cm–1 (Fig. 1, curve 3) is enhanced, intensity of absorption peak at about 3400 cm–1 (–OH) is shifted and enhanced, characteristic absorption peaks of epoxy groups almost disappeared in the spectrum of the gel, indicating the addition reaction of epoxy group with amine would progress successfully. The characteristic absorption peak of P–O in the pure CPO sample in Fig. 1 (curve 4) located at 1045 cm–1, while the absorption peak of P–O in PEI-PEGO-CPO gel in Fig. 1 (curve 5) shifted to 1095 cm–1, indicating the generation of N–H–O structure between PEGO-PEI and CPO, and the cross-linking of CPO to PEGO-PEI.
1H NMR Spectroscopy
The chemical structure of PEI-PEGO-CPO was also characterized using 1H solid state NMR spectra (Fig. 2). Influenced by inorganic CPO, the hydrogen peaks of –NH2, –NH group moved to 33.46–33.38 ppm, δH = 9.16–8.40 ppm is the peak of –OH group, δH = 9.16–8.40 ppm, the signal located at δH = 7.72–7.17 ppm corresponds to the oxyethylene unit protons (–CH2–CH2–O–), δH = 6.20 ppm corresponds to protons (–CHOH–), δH = 5.87 ppm corresponds to protons of –CH3.
Synthesis of PEGO-PEI by Means of Ring Opening Addition Reaction of PEI and PEGDE
Ring-opening addition reaction of PEI and PEGO was successfully conducted in H2O and produced the gels at room temperature without catalyst. An inflection point of the profile was defined as the gel formation time (not theoretical gelation time), summarized in Table 1. The gelation was not formed when the ratio of PEI and PEGO is 1 : 3 and 1 : 4 as the molecular weight of PEI and PEGO is not large enough after grafting. Hence, it does not form a three-dimensional network structure and no phase change was occurred. The gelation was not formed either when the ratio of PEI and PEGO is 1 : 8 as the polymer solution contains a large amount of PEGO and prevents the grafting reaction between PEI and PEGO. Therefore, the ratio of 1 : 5 between polyethyleneimine and PEGO intermediates is the optimal in terms of gelling effect.
Based on these results, inorganic stimuli-responsive hydrogel PEI-PEGO-CPO was synthesized under exactly the same conditions as those for the synthesis of PEGO-PEI via addition of 0.1% CPO to the system.
In addition, the effects of CPO on viscosity, temperature-sensitive property, pH-responsive property, and salt resistance of the gel were also investigated.
Effect of CPO on Temperature-Sensitive Properties of PEI-Based Gels
The molar ratio of PEI to PEGO is 1 : 5, the reaction temperature is 45°C, the total monomer mass concentration is 8%, and 0.1% CPO is added to the system to form hydrogels. The prepared hydrogels were then dried and placed in distilled water at different temperatures until the gel mass remained unchanged and the equilibrium swelling rates (ESR) at the different temperatures were measured.
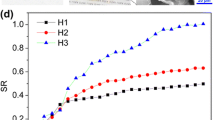
It can be seen from Fig. 3 that as the temperature increases, the gel swelling ratio of all the formulations gradually increases. Compared with PEGO-PEI, PEGO-PEI-CPO has a higher ESR. The presence of hydrophobic groups or structures with strong hydrogen bonds with water molecules are the common features of temperature-responsive polymers. For PEGO-PEI, the hydrogen bonds are formed between hydrophilic groups –CH2CH2O, –NH, –NH2 and water molecules. That will cause the hydrogel has good swelling properties at lower temperature [10]. Upon increasing temperature, the hydrogen bondings are weakened, the hydrated structures are gradually destroyed, which causes a lower ESR. On the other hand, with the increase of temperature, the molecular chain movement intensifies, so that the network structure of the gel can accommodate more water molecules, and at the same time, the water molecules can diffuse into the gel system more easily. Combining the influence of two factors, the ESR of the gel increases. CPO is bonded with PEI by hydrogen bonding [8], and the PEGO-PEI-CPO gel has more pores, which improves the swelling rate of the gel.
Effect of CPO on pH-Sensitive Properties of PEI-Based Gels
For the pH-responsive properties, glacial acetic acid and dilute NaOH solution were used to adjust the pH value of the resulting hydrogels (pH = 8, 9, 10, 11, 12 and 13). Aqueous solutions of acid and base (500 mL) were prepared at these pH values. The corresponding dry gels were added into the solutions prepared at different pH values at room temperature until the swelling equilibrium was achieved, the resulting hydrogels were weighed and the equilibrium swelling ratio calculated.
As shown in Fig. 4, the larger the pH value of the solution, the smaller the ESR. Therefore, it can be indicated that the hydrogel has significant pH sensitivity. Since the gels contain amine groups which are protonated at low pH values, the gel exhibit hydrophilicity and swell upon water absorption. Electrostatic repulsion between identical charges of the hydrogels leads to larger grid spaces that can promote water absorption. However, the amino groups are easily converted into neutral in an alkaline media and the swelling rate of the hydrogels is low. Whereas, the ESR of PEGO-PEI-CPO was greater than that of PEGO-PEI. One explanation of the result is that the CPO in the reaction system should yield gels with high crosslinking density. The grafting of CPO to PEGO-PEI is through the formation of hydrogen bonds with amino groups, the protonation of amino groups is enhanced, so the swelling rate of both PEGO-PEI-CPO is greater than that of PEGO-PEI.
Temperature-Sensitive and pH-Sensitive Reversibility of PEGO-PEI-CPO Hydrogels
In Fig. 5, the changes in the ESR curves obtained for the hydrogels alternately soaked for 24 h at 20 or 60°C in distilled water, and a buffered solution at pH 8 and 13, respectively. The swelling shrinkage behavior of the hydrogels have a periodic reversible change, that is, the hydrogel exhibits the characteristics of low-temperature shrinkage and high-temperature swelling under the same solution environment. The hydrogels have greater equilibrium swelling rates at pH 8 than in an alkaline environment pH 13. This phenomenon is consistent with the phenomena mentioned in sections “Temperature-Responsive Properties” and “pH-Responsive Properties,” showing good temperature- and pH-responsive characteristics. The temperature and pH response range of this hydrogel are suitable for oil well operation conditions used in the Daqing oil field, so that it is possible for water plugging and profile control.
CONCLUSIONS
The network polymers with a CPO unit were successfully synthesized by reactions of PEI with PEGO in H2O at 45°C without a catalyst. The influence of CPO on PEI-based gels was investigated. The thermosensitive and pH responsive properties of hydrogels were studied by measuring the ESR of hydrogels at different temperatures and pH. The hydrogel was switched at temperatures between 20 and 60°C, and pH values between 8 and 13. The results show that the addition of calcium phosphate oligomer CPO can effectively improve the temperature sensitive and pH responsive properties of PEI-based gels. In addition, the hydrogel system showed excellent reversibility for swelling–deswelling characteristics.
REFERENCES
T. Isaka, M. Yoshida, M. Owada, and K. Toyoshima, Virology 65, 226 (1975).
Z. Liu, R. Xie, X. Ju, W. Wang, and L.-Y. Chu, CIESC J. 67, 202 (2016).
N. Naga, M. Sato, K. Mori, H. Nageh, and T. Nakano, Polymers 12, 2047 (2020).
N. Naga, E. Oda, A. Toyota, K. Horie, and H. Furukawa, Macromol. Chem. Phys. 207, 627 (2006).
N. Naga, Y. Kihara, T. Miyanaga, and H. Furukawa, Macromolecules 42, 3454 (2009).
N. Naga, S. Fujioka, D. Inose, K. Ahmed, H. Nageh, and T. Nakano, RSC Adv. 20, 60 (2020).
S. I. Stupp and P. V. Braun, Science 277 (5330), 1242 (1997).
Z. Tang, N. A. Kotov, S. Magonov, and B. Ozturk, Nat. Mater. 2, 413 (2003).
Y. Yu, Z. Mu, B. Jin, Z. Liu, and R. Tang, Angew. Chem., Int. Ed. 59, 2071 (2020).
X. Liu, G. Qu, Q. Yu, N. Zhang, L. Wang, and J. Wang, Polym. Sci., Ser. B 62, 400 (2020).
Funding
Thanks for the financial support of National Nature Science Foundation of China (project no. 51834005), Hainan province Science and Technology Special Fund (ZDYF2022SHFZ107), Study on Application of intelligent nano system oil displacement method in the deep sea low permeability reservoirs, Local efficient reform and development funds for personnel training projects supported by the central government, Study on nanosystem displacement method of tight reservoir in Daqing Oilfield, Applied Technology Research and Development Program Project in Heilongjiang Province (GA20A201), Open Project Fund of State Key Laboratory of Inorganic Synthesis and Preparative Chemistry Jilin University, Support Program for Research Talents in Chemical Engineering and Technology of Northeast Petroleum University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ling Wang, Wang, J., Song, Kp. et al. Preparation of Organo-Inorganic Hybrid Gel Based on Branched Polyethyleneimine Crosslinked with Polyethylene Glycol Diglycidyl Ether and Calcium Phosphate Oligomers. Polym. Sci. Ser. B 64, 707–714 (2022). https://doi.org/10.1134/S1560090422700452
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090422700452