Abstract
A novel hydroxyl-terminated block copolymer (ITPP) binder was prepared through an in-situ preparation method. The new binder having similar block structure as HTPE binder, without complex synthesis process to prepare HTPE prepolymer intermediate, reduces cost and optimizes the preparation process. Thus, it is expected to be used as binder of insensitive propellant. Infrared spectroscopy, low-field nuclear magnetic resonance, and uniaxial tensile testing were used to investigate the curing networks and mechanical properties of the binder. The crosslink density Ve increased with the increase of TMP content and R value. The ultimate tensile strength σm of the in-situ-prepared ITPP binder is 20.50 MPa and the percentage of breaking elongation εb is 743.47%. Additionally, in order to study the pot life of the in-situ-prepared ITPP binder, the rheological properties of the curing reactions were also studied. Finally, compared to HTPE binder, the in-situ-prepared ITPP binder’s strength and elongation increase by 694 and 276%, respectively. Besides, the in-situ-prepared ITPP binder has better process performance. This exciting result greatly enhances that the in-situ-prepared ITPP binder has great potential for application in rocket propellant formulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
HTPE propellant is a kind of solid propellant with hydroxyl terminated block copolyether (HTPE) as binder, which was developed by the United States in the 1990s to improve the characteristics of HTPB composite propellant insensitive ammunition [1‒4]. It is internationally recognized as the most suitable propellant to meet the requirements of insensitive munition (IM) properties and high performance.
The key technologies of HTPE insensitive propellant are HTPE binder and N-butyl-N-(2-nitroxyethyl)nitramine (Bu-NENA) plasticizer. HTPE is a block copolymer of polytetrahydrofuran and polyethylene glycol, which has good low temperature mechanical properties and compatibility with energetic nitrate plasticizer [5, 6]. Due to the high dielectric coefficient of electrostatic insulation of HTPE binder, the possibility of electrostatic ignition and the risk of detonation during the use and production of an HTPE propellant are greatly reduced [7, 8]. Therefore, the research of HTPE binder has been widely concerned. HTPE is generally prepared by living ring opening polymerization of polyethylene glycol (PEG) and polytetrahydrofuran (PTMG). However, the synthesis process has many disadvantages such as complex process, long synthesis time, difficult block structure control, high cost and low yield [9‒17]. Therefore, it is necessary to develop an insensitive propellant binder with low cost, simple process and excellent comprehensive performance.
Previous research has also assessed the application of in-situ HTPE binders in improving the mechanical properties of propellants. Chen et al. synthesized and characterized a novel in-situ-prepared HTPE binder and demonstrated a lowered glass transition temperature and better mechanical properties compared with HTPE binder. Compared to the HTPE propellant, the mechanical properties of the in-situ-prepared HTPE propellant are greatly improved [18]. The design idea of in-situ preparation method is as follows: the block structure of HTPE, PEG, and PTMG were used as mixed prepolymers, difunctional diisocyanate was used as chain extender and crosslinker was added. Because of using diisocyanate as a curing agent, crosslinking and chain extending reactions exist simultaneously during the curing process, thus the target binder with similar block network structure to HTPE binder can be prepared.
In the work reported herein, for further improve the mechanical properties of binders for in-situ preparation of insensitive propellants, low-molecular-weight PEG and PTMG were used as the mixed segments. IPDI was used as chain extender and curing agent. TMP was used as crosslinker. PEG/PTMG binder system was prepared in situ, which was named in-situ-prepared ITPP binder. Soft and hard segment block structures were precisely controlled during polymerization. And the mechanical strength, process performance, and other properties of the prepared binders were systemically studied. Furthermore, HTPE binder film was prepared and was used to be compared mechanical properties with in-situ-prepared ITPP binder film. The results showed that the mechanical properties of in-situ-prepared ITPP binder film were significantly improved and this novel binder has potential applications in the field of insensitive propellant.
EXPERIMENTAL
Materials
The details of the chemical reagents in this work and their parameters are as follows. PEG with an average relative molecular mass Mn of ~200 and average functionality of 2 was obtained from Xi Long Chemical Co., Ltd. PTMG was obtained from the Shanxi Province Chemical Research Institute with a number average molecular weight Mn of ~1000 and average functionality of 2. HTPE was purchased from Liming Research Institute of Chemical Industry (Henan, China) with an average relative molecular mass Mn of ~4600 and hydroxyl value of 32.26 mg KOH/g. Polyfunctional isocyanate (N-100) with a number-average molecular weight Mn of 725 and average functionality of 3.87 was obtained from the Liming Research Institute of Chemical Industry (Henan, China). High-purity (>99.5 vol %) isophorone diisocyanate (IPDI) was obtained from Tianjin Guangfu Fine Chemical Research Institute. Trimethylolpropane (TMP) was purchased from Beijing Chemical Works with a hydroxyl group content of 23.60 mmol/g. Triphenyl bismuth (TPB) (purity of 99%) was obtained from Shanghai Institute of Organic Chemistry (Shanghai Municipality, China) and was formulated into a 0.5 wt % solution with dioctyl sebacate (DOS) as the solvent. Dibutyltin dilaurate (T12) from Shanghai Institute of Organic Chemistry (Shanghai Municipality, China) was formulated into a 0.5 wt % solution with dioctyl sebacate (DOS) as the solvent. Dioctyl sebacate (DOS) was analytically pure and obtained from Tianjin Guangfu Fine Chemical Research Institute. Bonding agent LBA-278, terminal cyano, and hydroxy-substituted polyamine was provided by Luoyang Liming Chemical Research Institute. The PEG, PTMG, and HTPE binders mentioned above were vacuum dried at 60°C for 9 h.
Preparation Process
HTPE binder was prepared according to the following steps. Prepolymer HTPE was first blended uniformly at a stoichiometric ratio followed by the addition of the curing agent N-100 with 10 min of stirring for each. Then 0.3 wt % of the curing catalyst (the mass ratio of the TPB solution to the T12 solution was 3 : 1) was added, and the mixture was stirred for another 10 min to achieve uniform mixing. The final mixture was further stirred and vacuumed at a temperature of 40°C for 1 h to remove the air bubbles. Then it was poured into a polytetrafluoroethylene mold and cured in an incubator at 60°C for 7 d, and then placed in a desiccator for 24 h.
In-situ-prepared ITPP binder was prepared according to the following steps. Prepolymer PEG200 and PTMG1000 were first blended uniformly at a stoichiometric ratio of 1 : 1, then the crosslinking agents IPDI and TMP were added, and each crosslinking agent was stirred for 10 min. 0.3 wt % of the curing catalyst (the mass ratio of the TPB solution to the T12 solution was 3 : 1) was added, and the mixture was stirred for another 10 min to achieve uniform mixing. The final mixture was further stirred and vacuumed at a temperature of 40°C for 1 h to remove the air bubbles. Then it was poured into a polytetrafluoroethylene mold and cured in an incubator at 60°C for 7 d, and then placed in a desiccator for 24 h.
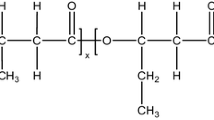
The sample preparation processes needed to be performed under ambient conditions with a temperature around 25°C and humidity values lower than 30%. Moreover, the processing conditions, such as the stirring time, also must be well controlled. The ratio of IPDI/TMP and R value (the curing parameter R denotes the equivalent ratio of isocyanate (‒NCO) to hydroxyl (‒OH) groups) are important design parameters for obtaining chemical crosslinking in the in situ-prepared ITPP binders. In the process of prepared in-situ-prepared ITPP binder samples, the molar content of TMP χ was varied in the range of 10–30% (the R value remains 1.3). The R values of HTPE and in-situ-prepared ITPP binder samples were varied in the range of 1.0–1.4. The χ and R values of the binder samples are listed in Table 1. The synthesis schemes for obtaining the in-situ-prepared ITPP binder and the HTPE binder are shown in Scheme 1a and 1b, respectively.

Scheme 1.
Analytical Methods
The mechanical properties were tested using a universal testing machine (AGS-J, Shimadzu, Japan) according to the method of GJB 770B-2005. Dumbbell-shaped samples were prepared and tested at 25°C and stretched at a rate of 100 mm/min. The dimensions of the test samples were 30 mm (neck position length) × 4 mm (width) × 2 mm (thickness). The crosslink density was examined by low field nuclear magnetic resonance spectroscopy (LF-NMR, VTMR20-010V-T, Niumai Corporation, China). The formation of the binder was conducted using Fourier transform infrared spectroscopy (FTIR, Nicolet 8700, Thermo Fisher Scientific, USA). These were performed with 48 scans in the middle infrared region with a spectral resolution of 2 cm–1 and spectral range 4000–500 cm–1, the samples were cut into 2 cm thick films for transmission spectroscopic analysis. Dynamic rheologies during the curing reaction of the samples were obtained using a rheometer (R/S-SST Plus, Brookfield) with a paddle rotor of 20–10 mm. A Helipath stand was used to prevent any channeling effects due to the spindle rotation. Viscosity measurements were made at 5 rpm and 60°C.
RESULTS AND DISCUSSION
Infrared Spectrum
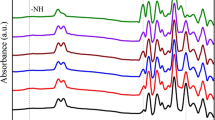
To confirm the formation of the in-situ-prepared ITPP binder, the chemical structure of the cured prepared binder was characterized by FTIR spectroscopy, and the results are shown in Fig. 1.
Figure 1 shows the infrared spectra generated by the in situ–prepared HTPE and the traditional HTPE binders. In the Fig. 1, the wide absorption band at 3327 cm−1 was caused by stretching vibrations of the associated amide NH groups. The peaks at 2939 and 2852 cm−1 are attributed to the C–H groups in the polymers (the peak at 2939 cm−1 corresponds to the C–H bond stretching vibration). The characteristic absorption peaks at 1725, 1526, and 1360 cm−1 correspond to amide I, amide II, and amide III, respectively. The characteristic absorption peak at 1105 cm–1 corresponds to the C–O–C bond of the polyether main chain. The stretching vibration of amide carbonyl (–C=O) bond appeared between 1735 and 1695 cm−1, proving the existence of a carbamate structure in the binder structure. The peaks related to O‒H groups and –NCO groups disappeared and new urethane groups appeared because of the interaction between hydroxy and isocyanate functionalities. These spectral data demonstrate that the HTPE-based PU binders were successfully synthesized.
Crosslinking Network
For thermosetting binders and propellants, the crosslinking network structure is the direct factor that affects the mechanical properties. The main parameters characterizing the structure of crosslinking network are crosslinking density and molecular weight between crosslinking points, which can be determined by low field nuclear magnetic resonance (LF-NMR) [19]. The different chemical environment and binding degree of hydrogen atom in polymer lead to different transverse relaxation time T2. The relaxation mechanism of hydrogen atom is more sensitive to the movement of molecular chain. Therefore, T2 of hydrogen atom can be used to characterize the movement or crosslinking degree of polymer chain. Literature research [20, 21] shows that XLD analysis model can be used as the regression model of crosslinking density. This paper uses this model to solve the crosslinking density. XLD model expression:
where M(t) is the amount of signal attenuation; A and B are the ratios of the signals corresponding to the crosslinked and tailing chain parts with respect to the signal of the entire polymer, respectively; A0 is the DC (direct current) component of the signal without explicit physical significance; T1 and T2 represent the relaxation times of the signals corresponding to the hanging tail chain and the crosslink, respectively; q is the anisotropy rate of the crosslinked part; Mrl is the residual dipole moment of the sample below the glassy temperature. After fitting the parameters with Eq. (1), the crosslinking density can be obtained according to Eq. (2):
where ρ is polymer density; N is the number of bonds in the backbone of the repeating unit; C is the number of bonds in the main chain in the Kuhn segment; M represents the molar mass of the repeating unit. T2 of the in-situ-prepared ITPP binder was carefully examined with the XLD model for regression analysis. The crosslink density and the molecular weight between the crosslinking points of each binder was calculated, as shown in Tables 2.
Table 3 shows that as the TMP content increased, the Ve of the binder gradually increased, while Mc gradually decreased. TMP is a trifunctional crosslinking agent, which mainly produces crosslinking effect. Therefore, as the TMP content increased, Ve gradually increases and Mc gradually decreases, which is conducive to improving the strength of the binder. As the R value of the in-situ-prepared ITPP binder increased, the Ve of the binder gradually increased, while Mc gradually decreased. The IPDI content increased with the increasing of R value, which is helpful to improve the conversion of curing reaction. And the excess curing agent can continue to react with carbamate group to form urea group, which increases the crosslinking degree of network structure. Therefore, the Ve of ITPP binder increased with the increasing of R value. Correspondingly, with the increased of crosslinking degree, the molecular weight Mc between crosslinking points decreased, so Mc decreased with the increasing of R value.
Figure 2 shows that at the same R value, compared with HTPE binder system, the crosslinking density of in-situ-prepared ITPP binder system is larger, but the change range is smaller. For HTPE binder, the curing agent is polyfunctional isocyanate N100, which is also a crosslinking agent. With the increase of R value, N100 crosslinking agent increases. However, for in-situ-prepared ITPP binder, the curing agent is only IPDI with bifunctionality. Therefore, with the increase of R value, the change range of crosslinking density and molecular weight between crosslinking points of in-situ-prepared ITPP binder system is slightly smaller.
Integrity of the Cross-Linked Network Structures
Hydrogen bond and crosslinking density are the microstructure factors of thermosetting binders. These factors have different effects on the network structure. How to comprehensively evaluate the advantages and disadvantages of a certain crosslinking network structure and how to reflect the mechanical properties from the perspective of network structure need to be further studied. Therefore, a semi quantitative and simple method is used to describe the integrity of the crosslinking network structure, which is helpful to analyze and adjust the mechanical properties.
The degree of integrity of in-situ-prepared ITPP binder network was studied by comparing the calculated and actual elastic modulus according to the rubber elasticity statistical theory of cross-linked structures [22]. The shear modulus G and the cross-linked network parameters have the following interrelations:
where G is the shear modulus; N is the total chain number in the crosslinked network; k is Boltzmann’s constant; T is the absolute temperature (298K); N' is Avogadro’s constant; ρ is the density of solidified network; Mc is the number average molecular weight between two adjacent crosslinking points; R is the molar gas constant (8.315 J/(mol K)).
But in practice, it is impossible to create such an ideal situation in the cross-linked structure because other network structures actually exist, mainly including hydrogen bonds and defects, such as entanglement, closed rings, side groups and free chains [23]. These structures can’t be accurately calculated and counted. Therefore, a correction factor D can be introduced to express their contribution to the shear modulus. Eq. (3) can be rewritten as:
where E is the elastic modulus; D is the correction factor. According to formula 4 and the existing data, the correction factor D in the in-situ-prepared ITPP binder film can be calculated. The results are listed in Table 3.
It can be seen from Table 3 that when TMP content is 15–30%, The correction factors of the in-situ-prepared ITPP binders are all positive values, indicating that the integrity of the cross-linked network of the ITPP binders is maintained under favorable conditions. Since the functionality of TMP is uniform, defects such as enclosed rings can hardly be formed after the curing reaction, which makes the network structure of ITPP binder more complete. Besides, with increasing TMP content, the shear modulus correction factor D increases first and then decreases. In the ITPP binder, with the increase of TMP content, the degree of hydrogen bonding first increased and then decreased, and Ve gradually increased. Bonded interactions and chemical cross-linking both contribute to the integrity of the cross-linked network structure, but excessive crosslinking density is not conducive to the entanglement of the molecular chain, which makes the structural integrity of the network worse. Thus, the correction factor D increases first and then decreases with the increase of TMP content, and the most complete curing network exists in the binder with the TMP content is 20%.
In addition, Table 3 shows that as the R value increases, the correction factor D increases first and then decreases. When R value is small, the reaction degree of the system is low, resulting in a large number of defects such as free chains and suspension chains, and the network structure is not perfect. Moderate increase of R value is beneficial to improve the conversion of curing reaction, reduce free chains and terminal defects, enhance bonded interactions, and improve the structure of crosslinking network. Therefore, D value increases gradually and reaches the maximum at R value of 1.3. However, when the ‒NCO group is surplus, which means the R value is too large, some of them continue to react with the urethane groups to form allophanate and enter the crosslinking network, while the other part self-polymerizes and form ring defects. Therefore, the network structure integrity will decline again, and the D value will gradually decrease. Comprehensive analysis shows that the network structure of binder is more complete when R value is 1.3.
Figure 3 shows the correction factors D of HTPE binders and in-situ-prepared ITPP binders at different R values. IPDI is both curing agent and chain extender in ITPP binder. When the R value is relatively small (1.1–1.2), compared with HTPE binder, ITPP binder has less –NCO groups in curing reaction, resulting in low degree of curing reaction and imperfect network structure. Therefore, the correction coefficient D of ITPP binder is lower than that of HTPE binder. When R value is large (1.3–1.4), the curing reaction degree of HTPE binder and ITPP binder is complete. The network structure of ITPP binder is relatively complete because the functionality of TMP is uniform and it is difficult to form a closed ring. Thus, the correction factor D of ITPP binder is higher than that of HTPE binder.
Mechanical Properties
The mechanical properties of binder determine whether it can be used in solid propellant. In ITPP binders, IPDI is a bifunctional curing agent, which can be used as chain extender of PEG and PTMG, while TMP is the only multifunctional component in the formula, which acts as a crosslinking agent in the crosslinking network. Obviously, the content of TMP affected the degree of curing reaction of the system, thus this paper explored the influence of TMP content on the mechanical properties of ITPP binder film. The reaction between isocyanate group (–NCO) and hydroxyl group (–OH) is the most basic reaction in the curing process of binder system. The R value is the molar ratio of –NCO/–OH, which determines the degree of curing reaction and is the key parameter affecting the performance of propellant binder. If R value is too small, some situations, such as incomplete curing reaction, low crosslinking density of binder film and poor mechanical properties, will occur. If R value is too high, the crosslinking density will be too high and side reaction will occur, which will seriously affect the curing quality and mechanical properties. Therefore, this paper explored the influence of R value on the mechanical properties of HTPE and ITPP binder polyurethane (PU), then compared the two properties to analyze the feasibility of the application of ITPP binders. Each sample was subjected to five repeated tests, and the tensile results shown in Fig. 5 correspond to the average of the five tests; the measurement error for the mechanical parameters is below 5.0%.
Figure 4a shows that with increasing TMP content, the σm increases and the εb decreases continuously. TMP is trifunctional and can be used as the center of cross-linking point when it takes part in curing reaction. With the increase of TMP content, the cross-linking points between molecular chains in the binder increase, which enhanced the force between molecular chains and made the ultimate tensile strength of the binder increased gradually. However, the increase of TMP content was reduced the molecular weight between crosslinking points of polymers, so the fracture elongation of binder film decreased. Considering the influence of TMP content on the σm and the εb of binder system, a sample with good comprehensive mechanical properties (TMP = 20%) was finally obtained.
Figure 4b shows that with increasing R value, the σm of in-situ-prepared ITPP binder system increases, while εb decreases. With the increase of R value, the isocyanate group increased, the crosslinking density increased, the molecular weight between crosslinking points decreased. Therefore, with the increase of R value, the tensile strength of the film increased. At the same time, with the increase of R value, the crosslinking density increased, the soft segment content between crosslinking points decreased, and the fracture elongation of polyurethane elastomer film decreased. By optimizing the formulation parameters of in-situ-prepared ITPP binder, the samples with good comprehensive mechanical properties (σm = 20.50 MPa, εb = 743.47%) were obtained, and the R value was 1.3.
Figure 5 shows that in the present work, when the R value is 1.3, the corresponding σm and εb values for the HTPE binder were 2.58 MPa and 197.43%. The σm and εb values of the in-situ-prepared ITPP binder were 7.94 and 3.76 times those of the HTPE binder, respectively. This improvement in mechanical properties is mainly because the special structure of the in-situ-prepared ITPP binders, there are more urethane bonds in the molecular chain. Because the polarity of the urethane is greater than that of the ether, the in-situ-prepared ITPP binders form hydrogen bonds more easily than the HTPE binders, and micro-phase separation occurs more easily. In addition, the crosslinking agent TMP of in-situ-prepared ITPP binder system has better structural symmetry than N100 of HTPE binder system. Therefore, the use of a mixture of TMP and IPDI as the crosslinking agent and curing agent yielded good mechanical properties. This interesting result demonstrates the feasibility of the in-situ preparation method and creates new possibilities for propellants.
Viscosity
Binder, whose technological properties are closely related to the technological properties of propellant, is the base of propellant. In the process of practical application, the binder is required to maintain a certain period of application to facilitate pouring. The standard period of application of the binder without solid filler is 25 Pa s. The proper viscosity can not only meet the process requirements, but also make samples better performance. The technological properties of HTPE binder and in-situ-prepared ITPP binder were studied.
Figure 6 shows the curve of the viscosity of the binder changing with the rate. The viscosity of in-situ-prepared ITPP binder system decreased with the increasing of shear rate, which belongs to non-Newtonian fluid. Compared with HTPE binder system, the in-situ-prepared ITPP binder system has lower viscosity and better process performance at the same rotational speed. The main reason is that the molecular weight of PEG and PTMG in the in-situ-prepared ITPP binder system is smaller than that of HTPE in the HTPE binder system.
Figure 7 shows the curve of binder viscosity changing with time. Under the isothermal condition, the viscosity of the binder system gradually increases with the extension of time. The application period of HTPE binder system is 5.6 h, and that of ITPP binder system is 15.1 h. Compared with HTPE binder system, the initial viscosity of ITPP binder system is lower, showing a low viscosity platform for a long time at the initial stage. And the viscosity of ITPP binder system increases slowly at the later stage. The application period of ITPP binder system is 15.1 h, which is mainly due to the low activity of IPDI used as chain extender in in-situ preparation of ITPP binder system. And the crosslinking agent is TMP, which has no curing effect. The premise of TMP crosslinking effect is that TMP needs to react with IPDI before entering the system, so the viscosity increases slowly, which makes the application period longer.
CONCLUSIONS
We synthesized and characterized a novel in-situ-prepared ITPP binder with PEG and PTMG functionalities. The crosslinking density of the in-situ-prepared ITPP binder exhibited a large range of variation, which increased first and then decreased with the increase of TMP content and R value. Based on the statistical theory of elasticity, the integrities of the curing networks were analyzed, showing that the TMP content and R value affected the integrity of the curing networks.
Uniaxial tensile tests were used to investigate in-situ-prepared ITPP binder mechanical performances. The mechanical properties were found to be significantly better than those of the traditional HTPE binder. This indicates that the in-situ-prepared ITPP has potential applications to replace the binder for HTPE propellants.
The statistical results showed that the pot life of in-situ-prepared ITPP binder met the composite propellant application requirements. Compared to HTPE binder, the in-situ-prepared ITPP binder’s strength and elongation increase by 694 and 276% respectively. Besides, the in-situ-prepared ITPP binder has better process performance.
REFERENCES
Q. F. Zhang and J. Q. Zhang, Energ. Mater. 12, 371 (2004).
K. O. Hartman and T. F. Comfort, in Proceedings of Insensitive Munitions and Energetic Materials Technology Symposium. Bristol,1996 (Bristol, 1996).
X. Q. Song, J. Y. Zhou, W. H. Wang, and X. H. Li, Chin. J. Energ. Mater. 16, 349 (2008).
D. Q. Yan, D. D. Xu, and J. G. Shi, J. Solid Rocket Technol. 32, 644 (2009).
W. B. Zhang, X. D. Fang, X. Z. Zhu, and W. W. Fan, J. Solid Rocket Technol. 2, 251 (2015).
C. D. Wang, Y. J. Luo, and M. Xia, Chin. J. Energ. Mater. 19, 518 (2011).
F. Kai, D. Tokerud, H. Biserod, E. Orbekk, S. Tenden, M. Kaiserman, M. Rodack, W. Spate, S. Winetrobe, B. Royce, and S. Wallace, in Proceedings of AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Tucson, AZ, USA, 2005 (Tucson, AZ, 2005), p. 4172.
M. Kaiserman, M. Rodack, W. Spate, S. Winetrobe, B. Royce, S. Wallace, H. Biserod, K. Fossumstuen, and D. Tokerud, in Proceedings of AIAA/ASME/SAE/ ASEE Joint Propulsion Conference and Exhibit, Tucson, AZ, USA, 2005 (Tucson, AZ, 2005), p. 4171.
A. F. Zhang, H. Z. Zhang, H. C. Yang, and X. D. Zhang, Chin. Sci. Bull. 35, 1881 (1990).
J. F. Neumer, US Patent No. 5 254 744 (1993).
G. Pruckmayr and R. B. Osborne, US Patent No. 5 284 980 (1994).
P. Holmqvist, A. A. Paschalis, and B. Lindman, Langmuir 13, 2471 (1997).
J. R. Goleniewski and J. A. Roberts, US Patent No. 5 783 769 (1998).
R. A. Barcock and R. J. Hobson, US Patent No. 5 773 207 (1998).
I. C. De Witte and E. J. Goethals, Polym. Adv. Technol. 10, 287 (1999).
C. Pomel, C. Leborgne, H. Cheradame, D. Scherman, A. Kichler, and P. Guegan, Pharm. Res. 12, 2963 (2008).
L. G. Niu, R. Nagarajan, F. X. Guan, L. A. Samuelson, and J. Kumar, J Macromol. Sci., Part A: Pure Appl. Chem. 43, 1975 (2006).
K. K. Chen, X. M. Wen, G. P. Li, S. P. Pang, and Y. J. Luo, RSC Adv. 10, 30150 (2020).
M. Garbarczyk, F. Grinberg, and N. Nestle, J. Polym. Sci., Part B: Polym. Phys. 39, 2207 (2001).
F. Zhao, P. Zhang, and S. G. Zhao, KGK Rubberpoint 5, 224 (2008).
W. Kuhn, E. Peregi, and Z. Fei, Mater. Res. Soc. Symp. Proc. 217, 33 (1991).
E. Fried, J. Mech. Phys. Solids 50, 571 (2002).
K. Z. Mao, S. Ma, and Y. J. Luo, Chin. J. Energ. Mater. 23, 941 (2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Yue Zhao, Chen, K. & Luo, Y. Isophorone Diisocyanate and Trimethylolpropane in-situ Prepared Hydroxyl-Terminated Block Copolymer Binder with Excellent Mechanical Properties. Polym. Sci. Ser. B 64, 382–392 (2022). https://doi.org/10.1134/S1560090422700117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090422700117