Abstract
To develop the colorless transparent fluorinated polyimide (PI) with low coefficient of thermal expansion (CTE) and high tensile strength, a series of ternary copolymerized fluorinated PIs were synthesized by 4,4-(Hexafluoroisopropylidene) diphthalic anhydride (6FDA), 2,2'-bis(trifluoromethyl) benzidine (TFMB), and the third monomer 3,3',4,4'-biphenyltetracarboxylic dianhydride (BPDA). The physical properties of the PI films were effectively regulated and optimized by adjusting the ratio of the rigid BPDA and flexible 6FDA components. By increasing the BPDA content, the thermal stability, dimensional stability, and mechanical properties of the polymers were enhanced. Among them, the PI film which molar ratio of 6FDA and BPDA was 5 : 5 exhibited an excellent comprehensive performance with a λ0 of 372 nm, Tmax nearly 91%, the tensile strength beyond 108 MPa and possessed high glass-transition temperature Tg which was 315.8°С. Furthermore, the CTE of the PI film was calculated about 21 ppm/K in the range of 25–200°С, indicating great dimensional stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Flexible electronics and flexible display technology are the most active research directions in the field of electronic information in recent years [1, 2]. Flexible display technology refers to the display technology that uses flexible material to replace fragile glass as a new substrate. The transparent flexible plastic substrates have been used in the next generation applications in the field of displays [3]. Of which, polyimide (PI) substrate material has attracted much attention due to its excellent high heat resistance, good mechanical properties, excellent chemical stability and other excellent properties [4, 5].
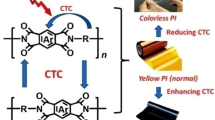
However, in PI molecule, it is easy to form charge transfer complexes (CTC), that make the traditional PI films appear brown and reduce their transmittance in the visible range [6]. As is well known, the fluorine atom has unique characteristics, such as high electronegativity and low electric polarity. Fluorinated PI can be synthesized by introducing fluorinated dianhydrides or fluorine-containing diamines. Fluorine atoms in the molecular chain can destroy the conjugated structure of the aromatic ring and inhibit the formation of CTC [7]. Therefore, fluorinated PI films have high transparent property, low refractive index, high thermal and chemical stability, great mechanical property, low water absorption and low dielectric constant [8, 9]. Fluorinated groups give PI films excellent performance, so that it is expected that fluorinated PI films will be widely applied in the photoelectric field and semiconductor industries [5, 10].
The introduction of fluorine or fluorine containing groups also bring some shortcomings, such as high coefficient of thermal expansion (CTE) and low mechanical property [11]. Specifically, introducing fluorinated groups increases the distance between molecular chains and free volume, hence, the CTE will be increased [12, 13]. Then, that also decreases the intermolecular interaction force, and destroys the molecular regularity. On one hand, although the solubility is increased, the decrease of mechanical properties is inevitable [14, 15]. On the other hand, the introduction of fluorine atoms will lead to a week adhesion with other substrates, which limits the development of fluorinated polyimide [16–18].
The copolymerization can change the molecular structure and chemical composition of the polymer and change its macro performance [19–22]. The copolyimides (Co-PIs) typically possess much lower molecular regularity than the corresponding homopolyimides. In order to increase the proportion of rigid benzene ring, the rigid rod, plane structure or short chain diamines and dianhydrides had been introduced into PI unit structure [23–26]. Furthermore, the properties of Co-PIs can be adjusted by varying the ratio of the comonomers, that was, by selecting the appropriate ratio of the dianhydride and diamine components. X. H. Yu [27] et al. have found that the CTEs of PI films obtained from the symmetrical aromatic diamine and biphenyl anhydride were the lowest. This kind of aromatic polyimide with rigid bar like structure is straighter, so the intermolecular piling is close, and it is beneficial to reduce the free volume of polymer and reduce the CTEs. For example, 3,3',4,4'-biphenyltetracarboxylic dianhydride (BPDA) is a good copolymerized monomer because it has symmetrical structure, chain arrangement, high stiffness and high reactivity. Moreover, the production technology of BPDA is mature and the cost is very cheap. After adding the BPDA, the properties of PI may be affected to different degrees, and the influence of different proportions is not the same, which is also one of the key points in this paper.
In this study, we intended to synthesize a series of fluorinated copolymerized PI (II). TFMB was used as diamines, 6FDA as fluorine-containing dianhydride and mixed with biphenyl dianhydride (BPDA), and the effect of fluorine atoms on the properties of PI was studied. In addition, on the basis of introducing fluorine-containing groups to improve the optical properties of the PI films, the effects of adding different proportions of BPDA copolymerization about the mechanical, optical, thermal and water absorption properties of PI films were investigated. It was hoped that the high-performance PI films with colorless transparency and low CTE can be obtained.
EXPERIMENTAL
Materials
4,4'-(Hexafluoroisopropylidene) diphthalic anhydride (6FDA), 3,3',4,4'-biphenyltetracarboxylic dianhydride (BPDA) were dried at 150°C for 8 h prior to use. 2,2'-bis(trifluoromethyl) benzidine (TFMB) was dried in a vacuum oven at 80°C for 8 h prior to use. N,N-dimethylacetamide (DMAC) was purified by distillation and purification prior to using. To improve the relative molecular weight of the polymers, all the reaction vessels, solvents, reactants must be strictly dried.
Synthesis
In the experiments, the precursors of polyimide (I series), which were polyamide acids (PAAs), were synthesized from the condensation of aromatic diamines and aromatic dianhydrides with [n(dianhydrides) : n(diamines) = 1.02 : 1], their ingredients were listed in Table 1. PAAs were completely imidized to PIs.
Take I-4 [n(6FDA) : n(BPDA) : n(TFMB) = 0.51 : 0.51 : 1] as an example. First, TFMB (3.2295 g) was added into a 50 mL three-neck flask, then 15 mL DMAC (solvent) was also added the same flask, and next we stirred them for about 0.5 h continuously in the 25°C until the diamine powder dissolved completely. After that, 6FDA (2.2848 g) and BPDA (1.5133 g) were added by three equal portions to the solution.Every time after adding the solid sample, the weighing paper must be flushed with 5 mL DMAC. After all the reagents were added, the temperature of the water bath can be adjusted to 8°C, and then continued to react for 24 h. In the process of mixing, the color of the reaction liquid deepened and the viscosity gradually increased, that was, the viscous PAAs solution formed. After the reaction was completed, the reaction liquid was put into the sealed bottle and stored at low temperature. Finally, the high viscosity PAAs solution was obtained with 20 wt % of total content.
The PAAs solution (I series) were evenly coated on the clean glass plates surface by the use of automatic coating drying machine, and the wet films’ thicknesses were controlled by scraper. The films placed in a vacuum oven accomplish imidation according to Fig. 1. Their temperature programs were, 90°C/1 h, 0 MPa and 120°C/1 h, –0.02 MPa, 150°C/1 h, ‒0.04 MPa, to removal of solvent. Then the semidried PAA films were placed in the muffle furnace and heated sequentially at 210°C/0.5 h, 240°C/0.5 h, 270°C/0.5 h, 300°C/1 h, respectively. After cooling to room temperature, the glass substrates were boiled in a water bath at 80°C. The PI films were uncovered and dried at room temperature. Finally, we got a series of fluorinated PI films (II series) and the thicknesses of PI films were about 25 μm.
Measurements
The apparent viscosities of the PAAs were determined by DV-2+PRO Digital viscometer at 30°C. IR spectra was recorded on a Shimadzu FTIR-8400S Fourier transform infrared spectrometer that provided qualitative information describing the conversion of the PAA to the PI. The test conditions were as follows: the number of scanning times was 20 times and the range of wave number was 4000–400 cm–1. 1H NMR spectrum was measured using a Bruker Ultrashield 300 MHz NMR spectroscopy in an ambient temperature. Test conditions: CDCl3 used as solvent, TMS as internal standard. The mechanical properties of PI films were measured by a SANS microcomputer controlled electronic universal testing machine. The test samples were made into ca. 0.025 mm thick, 80 mm wide, and 12.5 mm gauge length. The measurements were performed at room temperature and the actual thickness of the tensile specimen should be accurately measured by a spiral micrometer before the experiment, which can be used for tensile testing. Measurement conditions: the drawing speed was 20 mm/min, and the original standard distance was 20 mm. An average of at least three individual determinations was used. UV–Vis spectra of the polymer films were recorded on a Shimadzu UV-1600S spectrophotometer. The scanning wavelength range was 200–800 nm, and the distance was 1 nm. Thermogravimetric analysis (TGA) was performed with a DTG-60 system thermogravimetric analyzer of Shimadzu Corporation. Measurements were carried out on 3–5 mg film samples heated in flowing nitrogen (nitrogen gas flow velocity: 20 mL/min) and the temperature range was rising from room temperature to 800°C at a heating rate of 20 deg/min. Glass-transition temperatures Tg of polymers were determined on a TA Instruments DSC25 at the heating rate of 20 deg/min under nitrogen atmosphere. The CTEs of the films were measured by a TMA-402/F3 static thermomechanical analyzer of Germany Netzsch Company. Test conditions: the test temperature was 25–200°C, the heating rate was 10 deg/min, the charge was 50 mN. All the CTEs in this study were measured between 50–200°C. The contact angles against water of the II series PI films were recorded by a SL2008B Contact Angle Tester at room temperature. Water absorption was determined by the weighing of the changes in vacuum dried film specimens before and after immersion in deionized water at 25°C for 72 h. Water absorption (%) = (Ms – Md)/Md × 100%, where Ms and Md were the weights of swollen and dried film, respectively.
RESULTS AND DISCUSSION
The synthetic routes of II series PIs are shown in Scheme 1. In this paper, PIs were synthesized by the traditional two step method. The PAAs were first synthesized by three component copolymerization, and then the PI films were prepared by thermal imidization.

Scheme 1.
A colorless transparent polyimide film can be prepared by condensation and thermal imidization of fluorinated dianhydride (6FDA) with fluorinated diamine (TFMB). However, due to the introduction of fluorinated groups, the activity of monomers decreased, and the mechanical properties of PI films were poor compared to the usual Kapton type polyimide films [28]. In this experiment, the BPDA monomer with biphenyl structure was added to improve the mechanical properties of fluorinated PI. As we can see from Table 1, the viscosities of PAAs series were between 1024–42 360 cP. The viscosity of I-7 was 42 360 cP and almost 41 times of the I-1 whose viscosity was only 1024 cP. With the increase of BPDA monomer content, the apparent viscosity of PAAs showed upward trend which was mainly because the activity of BPDA monomer was obviously higher than that of 6FDA monomer.
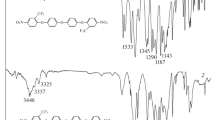
The infrared spectrums of all the PI films are shown in Fig. 2. It can be seen that there were no characteristic absorption peaks for PAA at 1650 and 1560 cm–1. At the same time, the characteristic peaks of polyimide appeared. For example, the characteristic peaks appeared near 1780 cm–1 were the asymmetric stretching vibration of C=O groups [29]. The characteristic peaks near 1729 cm–1 belonged to the symmetric stretching vibration of C=O group. The characteristic peaks near 1380 cm–1 belonged to the vibration of C–N group on the imine ring. The characteristic peaks near 725 cm–1 belonged to the bending vibration of C=O and these four kinds of characteristic absorption peaks were very sharp. Besides, the multiple peaks near 1100 to 1300 cm–1 belonged to the stretching absorptions of C–F groups [16]. It can be concluded that all the PAAs were completely imidized to PIs.
The 1H-NMR spectrum is shown in Fig. 3 of the typical PI II-4 derived from 6FDA with BPDA and TFMB monomers, was used as an example to confirm its molecular structure. All the aromatic protons resonated in the region of 7.51–8.32 ppm. The chemical shifts were in complete agreements with the proposed polymer structures. In addition, the complete imidization was also confirmed because no signal was observed above 10 ppm [30].
The tensile strengths of the II series PI films are shown in Fig. 4. It can be seen that the tensile strengths of PI films were between 83.8–117.5 MPa. When the molar fraction of BPDA monomer was between 0–50 mol %, the tensile strengths of the PIs increased with the increase of the content of BPDA monomer; when the molar content of BPDA monomer was 50–80 mol %, the tensile strengths decreased with the increase of the molar content of BPDA monomer. This was mainly due to the addition of low content but highly active BPDA monomer, which can increase the rigidity of the molecular chain, enhance the intermolecular force, and make the film exhibit tensile strength [31, 32]. When the content of BPDA monomer exceeded a certain range (>50 mol %), TFMB monomer reacted preferentially with BPDA monomer with high activity, which decreased the regularity of molecular chains and generated the too high rigid and fragile PIs, leaded to a decrease in the tensile strengths of PI films. When the content of BPDA monomer was 50 mol %, the tensile strength was as high as 108.9 MPa.
The mechanical properties and thermal properties of PI films were improved by introducing BPDA monomer with rigid biphenyl structure. However, the addition of high content of non-fluorine dianhydride BPDA monomer will reduce the optical transmittance and increase the color of the films. With the increase of BPDA monomer content, the UV–Vis curves of the corresponding copolymerized PI films moved to long wavelength, indicating that the increase of BPDA monomer will reduce the optical properties of the PI films. Therefore, we studied the optimum content of BPDA monomer, so that the copolymer PI films had excellent mechanical properties while maintaining excellent optical properties. The UV–Vis spectra of II series PI films are shown in Fig. 5.
The optical photographs of three types of PI films are also clearly observed in the Fig. 5. From top to bottom were the Kapton, II-1, II-4, and II-7 PI films, respectively. The II-1, II-4 and II-7 PI films were transparent and colorless compared with traditional Kapton PI film, indicating that they all had great optical transparency.
Their optical performance parameters are listed in Table 2. When BPDA monomer content increased from 0 to 100 mol %, λ0 increased from 339 to 384 nm, λT = 85% also increased from 405 to 519 nm, and at the same time, Tλ = 400 nm and Tλ = 500 nm decreased from 82.59 to 10.97% and from 90.17 to 85.13%, respectively. This was mainly due to the increase of BPDA monomer content and the decrease of 6FDA monomer content in copolymerized PIs, which leaded to the formation of CTC in PI molecular chains [33–35]. When n(6FDA) : n(BPDA) = 5 : 5, the λ0 of II-4 was 372 nm, Tλ = 500 nm was over 88%, and the Tmax was 91.35%.
Thermal properties of the PI films were evaluated by thermogravimetric analysis (TGA), differential canning calorimetry (DSC), and thermomechanical analysis (TMA).
The TG curves of II series are shown in Fig. 6, and the corresponding thermal performance parameters and specific figures are listed in Table 3. As follows from Fig. 6 and Table 3 II series synthesized by TFMB monomer with 6FDA and BPDA, showed poor heat resistance compared with the pure non-fluorine PI film, which was mainly due to the strong negative and large volume –CF3 which can destroy the conjugated structure of the molecules, reduce the interaction between the molecular chains, and lead to the decrease of thermal stability [36]. The T5% of II series were between 534.5~573°C, T10% were between 557.7~604.5°C, and residual mass Mre was more than 39.5% at 700°C. In the II series PI films we can find that the thermal stability of the copolymerized PI films increased with the increase of BPDA content. This was mainly due to the introduction of BPDA, which had effectively increased the rigidity of the molecular chain and enhanced the intermolecular force, thus effectively increased the thermal stability of the molecules [21].
The TMA test can record the temperature dependence of the volume, area and length of the material when it was heated. In addition, we can also calculate the CTE of the material through the TMA test. Generally speaking, the lower CTE, the better the dimensional stability of the material when heated. The CTE values of the II series are shown in Table 3.
Table 3 indicates that the CTE values decreased with the increase of rigid BPDA monomer content. For example, The II-4 of CTE value was only 21.0 × 10–6 K–1, and it had great thermal dimensional stability compared with Kapton films whose CTE was 47.0 × 10–6 K–1 [29]. The CTE of II series decreased from 47.9 × 10–6 to 13.7 × 10–6 K–1 when BPDA monomer content increased from 0 to 100 mol %. This was mainly because the large hexafluoroisopropyl group in 6FDA molecular structure will reduce the regularity of molecular chains and hinder the accumulation of molecular chains, while the rigid biphenyl structure in BPDA monomer was conducive to the accumulation of molecular chains.
The contact angles against water and water absorption of the resulting PI films are listed in Table 4. The contact angels and water absorptions of II series were in the range of 105.64–113.92° and 0.38–0.69% respectively. With the increase of BPDA content, the contact angle decreased and the water absorption increased, indicating that the hydrophobic performance became worse. This was because the introduction of rigid non-fluorine monomer (BPDA) reduced the content of strongly hydrophobic fluorine-containing groups.
CONCLUSIONS
A series of ternary copolymerized fluorinated PIs (II series) were synthesized by introducing rigid BPDA to investigate the effects of BPDA monomer content on the optical properties, CTEs, mechanical properties, and hydrophobic properties. The addition of BPDA can increase the viscosities of PAAs (I series), enhance the thermal stabilities and mechanical properties of fluorinated PI films, and also maintain the good optical transmittances. The CTEs decreased with the increase of rigid BPDA content. In particular, among all II series PI films, II-4 had the best comprehensive properties: high tensile strength, high optical transmittance, low CTE, etc. It indicated that the PI film had potential applications in the field of optoelectronics such as flexible display substrate and solar cell.
REFERENCES
J. M. Liu, T. M. Lee, C. H. Wen, and C. M. Leu, J. Soc. Inf. Disp. 19, 1 (2011).
H. R. Khaleel, H. M. Al-Rizzo, and D. G. Rucker, J. Disp. Technol. 8, 2 (2012).
W. J. Bae, M. K. Kovalev, F. Kalinina, M. Kim, and C. Cho, Polymer 105, 124 (2016).
S. D. Kim, B. Lee, T. Byun, I. S. Chung, J. Park, I. Shin, N. Y. Ahn, M. Seo, Y. Lee, Y. Kim, W. Y. Kim, H. Kwon, H. Moon, S. Yoo, and S. Y. Kim, Sci. Adv. 4, 10 (2018).
D. J. Liaw, K. L. Wang, Y. C. Huang, K. R. Lee, J. Y. Lai, and C. S. Ha, Prog. Polym. Sci. 37, 7 (2012).
K. H. Nam, W. Lee, K. Seo, and H. Han, Polymer (Korea) 38, 4 (2014).
C. P. Yang, Y. Y. Su, S. J. Wen, and S. H. Hsiao, Polymer 47, 20 (2006).
L. Tao, H. Yang, J. Liu, L. Fan, and S. Yang, Polymer 50, 25 (2009).
L. Li, Y. Xu, J. Che, X. Su, and C. Song, Polym. Bull. 75, 12 (2018).
H.-J. Ni, J. G. Liu, Z. H. Wang, and S. Y. Yang, J. Ind. Eng. Chem. 28, 16 (2015).
Z. Ge, L. Fan, and S. Yang, Eur. Polym. J. 44, 4 (2008).
I. H. Choi, B. Sohn, and J.H. Chang, Appl. Clay Sci. 48, 1(2010).
Y. Lu, J. Hao, G. Xiao, H. Zhao, Z. Hu, and T. Wang, Appl. Surf. Sci. 394, 78 (2017).
C. P. Yang and Y. Y. Su, Polymer 46, 15 (2005).
H. Matsutani, N. Arima, Y. Ishikawa, M. Matsunaga, and Y. Okada, J. Photopolym. Sci. Technol. 32, 3 (2019).
S. H. Hsiao, W. Guo, C. L. Chung, and W. T. Chen, Eur. Polym. J. 46, 9 (2010).
Y. I. Lee, and Y. H. Choa, J. Mater. Chem. 22, 2 (2012).
H. Li, J. Liu, K. Wang, L. Fan, and S. Yang, Polymer 47, 4 (2006).
C. H. Choi, B. H. Sohn, and J. H. Chang, J. Ind. Eng. Chem. 19, 5 (2013).
G. Song, S. Wang, D. Wang, H. Zhou, C. Chen, X. Zhao, and G. Dang, J. Appl. Polym. Sci. 130, 3 (2013).
R. Balasubramanian, S. S. Kim, and J. Lee, High Perform. Polym. 31, 9 (2018).
Y. Xu, L. Li, J. Che, and Z. Ye, J. Mater. Res. 34, 4 (2019).
H. S. Jin and J. H. Chang, J. Appl. Polym. Sci. 107, 1 (2008).
H. S. Jin, J. H. Chang, and J. C. Kim, Macromol. Res. 16, 6 (2008).
Z. Rafiee and M. Mohagheghnezhad, Polym. Bull. 76, 8 (2018).
W. Zhao, Y. Xu, C. Song, J. Chen, and X. Liu, e-Polym. 19, 1 (2019).
X. H. Yu, J. N. Liu, and D. Y. Wu, Mater. Today Commun. 21, 100562 (2019).
X. L. Zhang, C. Song, M.H. Wei, Z. Z. Huang, and S. R. Sheng, High Perform. Polym. 31, 8 (2018).
L. Li, Y. Xu, J. Che, X. Liu, W. Zhao, and Z. Ye, Macromol. Res. 27, 9 (2019).
F. Yang, Y. Li, T. Ma, Q. Bu, and S. Zhang, J. Fluorine Chem. 131, 7 (2010).
Y. Liu, Y. Zhang, Q. Lan, S. Liu, Z. Qin, L. Chen, C. Zhao, Z. Chi, J. Xu, and J. Economy, J. Chem. Mater. 24, 6 (2012).
X. Lei, M. Qiao, L. Tian, Y. Chen. and Q. Zhang, J. Phys. Chem. C 120, 5 (2016).
Y. Guan, C. Wang, D. Wang, G. Dang, C. Chen, H. Zhou, and X. Zhao, Polymer 62, 1 (2015).
C. Y. Guo, Q. W. Wang, J. G. Liu, L. Qi, M. G. Huangfu, X. Wu, Y. Zhang, and X. M. Zhang, eXPRESS Polym. Lett. 13, 8 (2019).
K. H. Kim, S. Jang, and F. W. Harris, Macromolecules 34, 26 (2001).
H. Yao, Y. Zhang, K. You, Y. Liu, Y. Song, S. Liu, and S. Guan, React. Funct. Polym. 82, 58 (2014).
Funding
The authors are grateful to the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zheng Zhu, Xu, Y., Ye, Z. et al. Synthesis and Properties of Colorless Transparent Polyimides with Low CTE and High Tensile Strength. Polym. Sci. Ser. B 62, 756–764 (2020). https://doi.org/10.1134/S1560090420330076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090420330076