Abstract
In this study, well-defined thermo-responsive block copolymers of poly(ε-caprolactone)-b-poly(N-isopropylacrylamide) with narrow molecular weight distribution were synthesized in one-step via reversible addition-fragmentation chain transfer (RAFT) polymerization and ring-opening polymerization (ROP) methods using dual RAFT-ROP initiator which was obtained via the reaction of 3-chloro-1,2-propanediol with the potassium salt of ethyl xanthogenate. Thermo-responsive block copolymers were obtained in high yield and high molecular weight. The characterization of the products was accomplished by NMR and FTIR spectroscopy, gel permeation chromatography, thermogravimetric analysis, differential scanning calorimetry. Furthermore, thermo-responsive properties of the copolymers were verified by UV, and fractional precipitation techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Smart polymers such as stimuli-responsive materials make up the majority of the polymer industry since they can sense and respond to the changes in environmental conditions such as pH, temperature, light, CO2, and magnetic field or electrical fields. One of the most important smart polymers is thermo-responsive polymers [1]. Thermo-responsive amphiphilic copolymers show changes in shape or size of the coil above the lower critical solution temperature (LCST) of the polymers due to swelling or shrinking of the thermo-responsive part that causes a changes in the hydrophilic–hydrophobic balance [2–4]. These materials usually go through a volume transition or soluble and insoluble changes in solvents near the LCST. One of the most commonly studied thermo-responsive polymers is based on poly(N-isopropylacrylamide) (PNIPAM) because it has an LCST of 32°C in water, which is close to the human body temperature [5]. Recently, the interest in PNIPAM has greatly increased due to its promising potential in a number of applications such as drug delivery systems, separation and purification and in the fields of nanotechnology and bioengineering [6, 7].
With the emergence of new living polymerization techniques, such as nitroxide-mediated polymerization, atom transfer radical polymerization and reversible addition-fragmentation chain transfer (RAFT) polymerization, NIPAM containing thermo-responsive polymers with well-defined structure have been synthesized. Huanhuan Liu et al. carried out the synthesis of PCL-b-PNIPAM-b-PAA star graft copolymers through the one-step combination of ROP, RAFT and ATRP polymerization [8]. Various copolymers containing L-lactide, N-isopropylacrylamide [9] and glycerol, N-isopropylacrylamide [10] monomers were also synthesized by a combination of the RAFT and ROP methods. Controlled polymerization strategies have facilitated the synthesis of a number of interesting complex branched block and graft copolymers which have narrow molecular weight distribution and a well-defined structure because all chains are growing at equal rates, or in other words, the molecular weight increases linearly with conversion [11–13]. Controlled radical polymerization techniques are based on radical closing mechanisms and agents [14, 15]. After intensive studies on ionic-living polymerization techniques, it has recently become important to develop free-radical polymerization that can demonstrate the characteristics of living polymerization techniques [16]. RAFT polymerization represents the most recently developed controlled radical-polymerization method and is a powerful technique for the macromolecular synthesis of a broad range of well-defined polymers. The flexibility of this polymerization method can be verified with a wide range of monomer and reaction conditions [17–30].
Well-defined synthesis of block copolymers is a significant topic in macromolecular chemistry. Block copolymers that provide particular combinations of physical properties are the appropriate materials for different purposes [31]. Macroinitiators have been widely used for designing diverse block copolymers via a radical set of processes [32, 33]. Synthesis of block copolymers has recently been successful with the combination of different techniques. Block copolymers which have excellent physical properties are one of the most important polymeric materials used in technological applications and theoretical research because of their exceptional properties based on micro-phase separation. Block copolymer is largely used as resistant materials. There are a large number of superior articles published on this subject [34–38].
In this study, the thermo-responsive block copolymers poly(ε-caprolactone)-b-poly(N-isopropylacrylamide) with narrow molecular weight distribution and long block lengths were synthesized in one-step in the presence of RAFT-ROP initiator.
EXPERIMENTAL
Materials
The potassium salt of ethyl xanthogenate, 3-chloro-1,2-propanediol, NIPAM, 2,2′-azobisisobutyronitrile (AIBN), THF, n-hexane, benzene and diethyl ether were received from Aldrich and used as received. Dibutyltin dilaurate (DBTDL) and ε-caprolactone (CL) were supplied by Merck and used as received. All other chemicals were reagent grade and used as received.
Characterization
The molecular weights and molecular-weight distributions were measured with Polymer Labs PL-GPC 220 gel-permeation chromatography (GPC) with THF as the eluent. A calibration curve was generated with three polystyrene standards: 2960, 50 400, and 696 500 Da, of low polydispersity. Fourier-transform infrared spectroscopy (FTIR) spectra were recorded with a Perkin Elmer Spectrum 100 Model FTIR spectrometer in transmissive mode and scan rate 400 to 650 cm−1. 1H NMR spectra of the samples in CDCl3 as the solvent, with tetra methylsilane as the internal standard, were recorded using a Bruker Ultra Shield Plus, ultra-long hold time 400 MHz NMR spectrometer.Thermal analysis measurements of the polymers were carried out under nitrogen using a Perkin Elmer Pyris 1 TGA and Spectrum thermal analyzer to determine thermal degradation. Thermo-responsive properties of the copolymers were verified by UV–Vis spectrophotometers SHIMADZU-1240.
Synthesis of Dual RAFT-ROP Initiator
For the synthesis of dual RAFT-ROP initiator, 2.0 g of 3-chloro1,2-propanediol, 5.0 g of the potassium salt of ethyl xanthogenate and 30 mL of THF as solvent were charged in a 20 mL vial under nitrogen gas. The mixture was stired at 25°C for 72 h on a magnetic stirrer. Then, the solution was filtered to remove the unreacted xanthate, and the solvent was removed by a rotary evaporator. The RAFT-ROP initiator was precipitated in cold diethyl ether and dried under vacuum at room temperature for four days.
Synthesis of Poly(ε-caprolactone)-b-poly(N-isopropylacrylamide) Thermo-Responsive Block Copolymers by One-Step Polymerization (RAFT-ROP)
For the RAFT-ROP polymerization of poly[CL-b-NIPAM] thermo-responsive block copolymers, particularized quantites of the RAFT-ROP initiator, NIPAM, CL, AIBN, DBDTL (catalyst for ROP of CL), and benzene (as solvent) were placed into a tube under nitrogen gas. The amounts of chemicals used in the polymerization are shown in Tables 1–3. The tube was tightly capped with a rubber septum and was dropped into an oil bath thermostated at 80°C for fixed temperatures. After the polymerization, the reaction mixture was flowed into an excess of n-hexane to precipitate poly(CL-b-NIPAM) thermo-responsive block copolymers. The polymers were dried at 25°C under vacuum for three days. The yield of the polymer was determined gravimetrically.
Fractional Precipitations of the Polymers
Fractional precipitations of the polymers were carried out according to the procedure described in literature [39, 40]. Vacuum-dried polymer sample (approximately 0.5 g) was dissolved in 5 mL of THF. Petroleum ether was added drop wise as a nonsolvent to the solution while stirring until turbidity occurs. At this point, 1–2 mL of nonsolvent was added to complete the precipitation. The precipitate was removed by filtration. The γ values were calculated as the ratios of the total volume of nonsolvent used for the first fraction to the volume of solvent used:
The nonsolvent addition into the filtrate solution was continued according to the same procedure mentioned above to determine the γ value for the second fraction if any.
Thermo-Responsive Properties of Block Copolymers
In a typical example, 2 mg poly(CL-b-NIPAM) thermo-responsive block copolymers was dissolved in 10 mL water. Primarily, the transmittance values (T%) of the solutions were obtained by UV measurement at 600 nm from 4 to 42°C.
RESULTS AND DISCUSSION
Synthesis of RAFT-ROP Initiator
The dual RAFT-ROP initiator was synthesized through the reaction of 3-chloro1,2-propanediol with the potassium salt of ethyl xanthogenate. The yield of initiator was nearly 76.5%. The synthesis mechanism for dual RAFT-ROP initiator is given on Scheme 1.

Scheme 1.
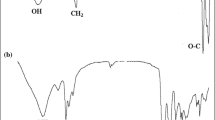
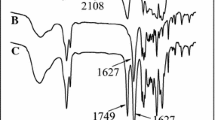
The FTIR spectra of 3-chloro1,2-propanediol in Fig. 1 (curve 1) shows the bands at 3434 cm–1 for –OH groups, 2890 cm–1 for aliphatic –CH2 and –CH groups and 1060 cm–1 for –O–C groups. The FTIR spectra of initiator in Fig. 1 (curve 2) also indicates the characteristic bands of –OH groups at 3434 cm–1, ‒CH2 and –CH groups at 2780 cm–1, –C=S groups at 1600 cm–1 and –O–C groups at 1060 cm–1. The signal of –C=S groups (1600 cm–1) at the spectra of the RAFT-ROP initiator (Fig. 1, curve 2) were not observed at the spectra of 3-chloro1,2-propanediol (Fig. 1, curve 1).
The 1H NMR spectrum of 3-chloro1,2-propanediol in Fig. 2a shows the 3.5 ppm for –OH protons, 3.7 ppm for –CH2–Cl protons, 3.9 ppm for –CH and CH2 protons. The 1H NMR spectrum of RAFT-ROP initiator in Fig. 2b shows 1.1 ppm for aliphatic –CH3 protons of ethyl xanthogenate segment, 1.6 ppm ‒CH2 and –CH protons of 3-chloro1,2-propanediol segment, 2.2 ppm –OH protons of 3-chloro1,2-propanediol segment, and 3.7 ppm –CH2 protons of 3‑chloro1,2-propanediol segment, and 3.9 ppm ‒OCH2 protons of ethyl xanthogenate segment.
Synthesis of Poly(ε-caprolactone)-b-poly(N-isopropylacrylamide) Thermo-Responsive Block Copolymers by One-Step Polymerization (RAFT-ROP)
The one-step polymerization of a thermo-responsive monomer and a lactone carried out in the presence of RAFT-ROP initiator is shown in Scheme 2. This polymerization creates three new active sites— two sites on an equal number of hydroxyl groups for the ROP reaction and one on the thiocarbonate group for RAFT polymerization. During the one-step synthesis, RAFT polymerization of the thermo-responsive monomer (NIPAM) was carried out simultaneously as the ROP of CL proceeds, to yield the thermo-responsive block copolymer. Thermo-responsive block copolymers, poly(CL-b-NIPAM) were obtained by RAFT-ROP method. These smart polymers have potential field applications and new application sectors, bioengineering and medicine. The results of the one-step polymerization of NIPAM and CL are shown in Tables 1–3. The monomer conversion was calculated from the weight of recovered polymer. The increases in the molecular weights of the copolymers the presence of the RAFT-ROP initiators confirms the formation of block copolymers.

Scheme 2.
The FTIR spectra of thermo-responsive block copolymer is given in Fig. 1 (curve 3). The bands shown at 3200 cm–1 can be assigned to –NH groups, 2600 cm–1 to aliphatic –CH2 and –CH groups, 1728 cm–1 to –C=O groups, and 1060 cm–1 to –O‒C groups. According to the 1H NMR spectrum of thermo-responsive block copolymers, Fig. 3 demonstrates 1.1 ppm for aliphatic –CH3 protons of ethyl xanthogenate and poly-NIPAM segment, 1.8 ppm for –CH2 and –CH protons of poly-NIPAM and 3-chloro1,2-propanediol segment, 1.9 ppm for –CH protons of poly-NIPAM segment, 2.7 ppm for ‒OCH2 protons of poly-CL and ethyl xanthogenate segment, 4.0 ppm for –OH protons of poly-CL segment, and 4.2 ppm for –NH protons of poly-NIPAM segment.
In this study, the impact of polymerization time, initiator amount, and monomer amount on the one-step copolymerization in the presence of the RAFT-ROP initiator by the application of simultaneous RAFT and ROP processes was evaluated. The impact of the polymerization time on the one-step copolymerization is exhibited in Table 1. It has been observed that higher molecular weights are obtained in the copolymerization with longer durations. However, the value of Mn decreases after 3.5 h of the polymerization. Longer polymerization time causes higher polymer yields. These results are in good agreement with those stated by Heidenreich and Puskas [41] for the RAFT polymerization. Increasing the amount of monomers also causes an increase in both the yield and the molecular weights of the copolymers as expected (Table 2).
Increased amounts of initiators in the reaction mixture lead to the formation of a higher number of active centers. Consequently, increased numbers of growing radicals are formed in the system. Hence, it may be expected that they have shorter poly-NIPAM and poly-CL segments, which is confirmed by a decrease in the molecular weights of the block copolymers, as shown in Table 3. The same situation was also observed in our previous studies [13, 29, 30].
The Mw/Mn values of the poly(CL-b-NIPAM) thermo-responsive block copolymers were between 1.06 and 2.20 (Tables 1–3). Due to the branched structure, more than one propagating center initiateds the polymerization, and the Mw/Mn values of the block copolymers were relatively higher than expected.
Block lengths were calculated using 1H NMR spectrum of thermo-responsive block copolymers and block length ratios are given in Tables 1–3. The polymer composition of the copolymers was calculated using the integral ratios of the signals corresponding to the –NH groups of poly-NIPAM (δH = 4.2 ppm), ‒OCH2 groups of poly-CL (δH = 2.7 ppm). The block length of the block copolymers can be adjusted by varying the amount of monomer and initiator and the polymerization time.
Thermal Analysis of poly(CL-b-NIPAM) Thermo-Responsive Block Copolymers
Thermal properties of thermo-responsive block copolymers were studied using DSC to determine glass transition temperatures Tg and using thermogravimetric analysis (TGA) to determine the decomposition temperatures Td. TGA showed interesting properties of the block copolymer indicating continuous weight loss starting from 10 to 1000°C. In the case of PCL-b-PNIPAM thermo-responsive block copolymer (BA-6), PCL, PNIPAM blocks had individual decomposition temperatures as shown in Fig. 4 (ca. 220 and 380°C, respectively).
TGA curves of poly(CL-b-NIPAM) thermo-responsive block copolymers (BA-6 in Table 1).
DSC analysis of thermo-responsive block copolymers shown in Fig. 5. Block copolymer (BA-6 in Table 1) exhibited two Tg values at 12.7 and 81.6°C, respectively. These values diasccord to the literature data of glass transition temperatures of corresponding hopolymers [42–44]. The obtained result may be due to the miscibility of the blocks of homopolymers.
DSC curve of the block copolymer (BA-5 in Table 1).
With the UV measurements of the thermo-responsiveness of block copolymers, the transmittance values (T%) at various temperatures, were determined at 600 nm (Fig. 6). The LCST of obtained block copolymers (BB-3, BB-4, BB-5) is about 31–33°C due to the presence of hydrophilic groups (–CONH–) and hydrophobic groups (–CH(CH3)2) [45]. The samples BB-1, BB-2 due to high content of hydrophilic units of PCL have no thermo-responsive properties.
Fractional Precipitation
Fractional precipitation experiments are an old technique used to prove the formation of block copolymers. In our previous studies, this method was used to prove the formation of copolymers [13, 15, 30]. The fractional precipitation values γ of poly(CL-b-NIPAM) thermo-responsive block copolymers were between 0.50 and 1.20, as shown in Tables 1–3. In the solvent–nonsolvent system, the γ-values were found to be homo poly-CL [31] 0.94–1.00, for homo poly-NIPAM 0.70–1.40. The γ values of the block copolymers were different from those of homo poly-CL, and homo poly-NIPAM. Fractional precipitation behavior gives an evidence for the formation of block copolymers.
CONCLUSIONS
In this study, poly(CL-b-NIPAM) thermo-responsive block copolymers were synthesized in one step by the RAFT-ROP method. By changing the initiator concentrations, monomer concentrations and polymerization time, the block copolymers compositions can be adjusted. The block copolymers (BB-3 – BB-5) exhibit thermo-responsive properties.The LCST of NIPAM and block copolymers (BB-3, BB-4, BB-5) are about 31–33°C. These thermo-responsive block copolymers can be applied to various areas such as new types of adaptive surfaces, sensors, antibacterial materials.
REFERENCES
N. Kalva and A. V. Ambadea, Polym. Int. 66, 1084 (2017).
C. Pietsch, U. Mansfeld, C. Guerrero-Sanchez, S. Hoeppener, A. Vollrath, and M. Wagner, Macromolecules 45, 9292 (2012)
W. Yuan, W. Guo, and G. Ren, Polym. Chem. 4, 3934 (2013).
Z. Cao, H. Wu, J. Dong, and G. Wang, Macromolecules 47, 8777 (2014).
P. Nuntahirun, O. Yamamoto, and P. Paoprasert, Polym. Bull. 75, 1387 (2018).
N. Qiu, Y. Li, S. Han, G. Cui, T. Satoh, T. Kakuchi, and Q. Duan, J. Photochem. Photobiol., A 283, 38 (2014).
S. Allı, A. Allı, and B. Hazer, J. Appl. Polym. Sci. 124, 536 (2012).
H. Liu, D. Tang, R. Tang, and Y. Zhao, Sci. China: Chem. 58, 1724 (2015).
W. A. Zhang, S. H. Wang, X. H. Li, J. Y. Yuan, and S. L. Wang, Eur. Polym. J. 48, 720 (2012).
P. Mugang, W. Decheng, and H. Junlian, Chin. J. Chem. 28, 499 (2010).
M. Nasiri, D. J. Saxon, and T. M. Reineke, Macromolecules 51, 245 (2018).
J. Zhang, D. K. Schneiderman, T. Li, M. A. Hillmyer, and F. S. Bates, Macromolecules 49, 9108 (2016).
T. Öztürk, M. Göktaş, and B. Hazer, J. Appl. Polym. Sci. 117, 1638 (2010).
Y. Wi, K. Lee, B. Hyung, and S. Choe, Polymer 49, 5626 (2008).
M. Göktaş, T. Öztürk, M. N. Atalar, A. T. Tekeş, and B. Hazer, J. Macromol. Sci., Part A: Pure Appl. Chem. 51, 854 (2014).
J. Hong, Q. Wang, and Z. Fan, Macromol. Rapid. Commun. 27, 57 (2006).
G. Moad, E. Rizzardo, and S. H. Thang, Polymer 49, 1079 (2008).
J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, E. Moad, E. Rizzardo, and S. H. Thang, Macromolecules 31, 5559 (1998).
D. H. Han and C. Y. Pan, J. Polym. Sci., Part A: Polym. Chem. 5, 789 (2007).
Y. K. Chong, J. Krstina, T. P. T. Le, G. Moad, A. Postma, E. Rizzardo, and S. H. Thang, Macromolecules 36, 2256 (2003).
S. Diaz-Silvestre, E. Saldivar-Guerra, C. Rivera-Vallejo, C. St Thomas, J. Cabello-Romero, R. Guerrero-Santos, and E. Jimenez-Regalado, Polym. Bull. 75, 891 (2018).
D. L. Patton and R. C. Advincula, Macromolecules 39, 8674 (2006).
J. J. Gallagher, M. A. Hillmyer, and T. M. Reineke, ACS Sustainable Chem. Eng. 4, 3379 (2016).
M. Le Hellaye, C. Lefay, T. P. Davis, M. H. Stenzel, and C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem. 46, 3058 (2008).
C. Cheng, E. Khoshdel, and K. L. Wooley, Macromolecules 40, 2289 (2007).
H. Mori, S. Masuda, and T. Endo, Macromolecules 41, 632 (2008).
C. Chang, H. Wei, C. Y. Quan, Y. Y. Li, J. Liu, Z. C. Wang, S. X. Cheng, X. Z. Zhang, and R. X. Zhuo, J. Polym. Sci., Part A: Polym. Chem. 46, 3048 (2008).
B. Luan, B. Q. Zhang, and C. Y. Pan, J. Polym. Sci., Part A: Polym. Chem. 44, 549 (2006).
T. Öztürk, O. Kayğın, M. Göktaş, and B. Hazer, J. Macromol. Sci., Part A: Pure Appl. Chem. 53, 362 (2016).
T. Öztürk, M. Yavuz, M. Göktaş, and B. Hazer, Polym. Bull. 73, 1497 (2016).
T. Öztürk, M.N. Atalar, M. Göktaş, and B. Hazer, J. Polym. Sci., Part A: Polym. Chem. 51, 2651 (2013).
T. Öztürk and B. Hazer, J. Macromol. Sci., Part A: Pure Appl. Chem. 47, 265 (2010).
T. Öztürk, M. Göktaş, B. Savaş, M. Işıklar, M. N. Atalar, and B. Hazer, e-Polym. 14, 27 (2014).
A. Watts, N. Kurokawa, and M. A. Hillmyer, Biomacromolecules 18, 1845 (2017).
B. Hazer, Eur. Polym. J. 27, 975 (1991).
T. Öztürk, M. Göktaş, and B. Hazer, J. Macromol. Sci., Part A: Pure Appl. Chem. 48, 65 (2011).
Z. Li, M. A. Hillmyer, and T. P. Lodge, Macromolecules 37, 8933 (2004).
D. Roy, J. T. Guthrie, and S. Perrier, Macromolecules 38, 10363 (2005).
T. Öztürk and E. Meyvacı, J. Macromol. Sci., Part A: Pure Appl. Chem. 54, 575 (2017).
B. Hazer, B. Erdem, and R. W. Lenz, J. Polym. Sci., Part A: Polym. Chem. 32, 2603 (1994).
A. J. Heidenreich and J. E. Puskas, J. Polym. Sci., Part A: Polym. Chem. 46, 7621 (2008).
M. Shuster, M. Narkis, and A. Siegmann, Polym. Eng. Sci. 34, 1613 (1994).
J. C. Huarng, K. Min, and J. L. White, Polym. Eng. Sci. 28, 1590 (1988).
R. G. Sousa, W. F. Magalhaes, and R. F. S. Freitas, Polym. Degrad. Stab. 61, 275 (1998).
R. Yoshida, K. Uchida, Y. Kaneko, K. Sakai, A. Kikuchi, Y. Sakurai, and T. Okano, Nature 374, 240 (1995).
Funding
This work was supported by the Yüzünci Yıl University Scientific Research Fund (grand number: FBA-2016-5036).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Göktaş, M., Olgun, B. One-Step Synthesis and Characterization of Poly(ε-caprolactone)-b-poly(N-isopropylacrylamide) Thermo-Responsive Block Copolymers via RAFT and ROP Techniques. Polym. Sci. Ser. B 61, 421–429 (2019). https://doi.org/10.1134/S1560090419040055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1560090419040055