Abstract
A technique for the experimental determination of the thermophysical characteristics of inactive aluminophosphate and borosilicate glasses by differential scanning calorimetry (DSC) is proposed and tested. The advantages of this measurement technique are shown. For glassy matrices of various compositions, the specific heat capacity and thermal conductivity coefficient are determined. The obtained experimental values make it possible to calculate the thermal conductivity values. The applicability of this method is proved by working with real samples of vitrified high-level waste to study their properties and form a database based on them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Management of radioactive waste accumulated as a result of the implementation of defense programs and during the processing of spent nuclear fuel is one of the most difficult environmental problems in modern Russia. The conversion of high-level waste (HLW) to a safe form of storage—solidification by vitrification—at Mayak Production Association remains a promising solution to this problem.
For the immobilization of HLW, two types of glasses are currently used: aluminophosphate (APS) and borosilicate (BSS). The basic composition of APS, as a rule, is represented by oxides of aluminum, phosphorus, and sodium, and BSS is represented by oxides of silicon, boron, and sodium. The glass composition is limited, on the one hand, by the solubility of the individual components of HLW in the glass, and, on the other hand, by the technological parameters of vitrification. Waste immobilization by vitrification is characterized by a relatively simple technology: glasses are boiled together with the simultaneous dosage of radioactive waste onto the surface of the melt. At Mayak Production Association, HLWs are cured in APS in electric furnaces of direct electric heating of the EP-500 type. In the future, it is planned to additionally use small melters of direct electric heating for curing in BSS.
To assess the possibility of the final isolation of vitrified HLW within the framework of cooperation with National Operator for Radioactive Waste management and substantiate the long-term stability of vitrified HLW intended to be returned abroad, the task of determining the thermophysical characteristics of various types of APSs and BSSs remains relevant.
In this paper, we propose and test a method for experimentally determining the thermal conductivity and specific heat of inactive APSs and BSSs by differential scanning calorimetry (DSC) on a synchronous thermal analysis instrument.
ANALYSIS OF EXPERIMENTAL METHODS FOR DETERMINING THERMAL CONDUCTIVITY
Thermal conductivity together with heat capacity is widely used for calculating and assessing the conditions for the safe storage of the obtained materials. Thermal conductivity is numerically equal to the product of the thermal conductivity of a substance and its specific heat capacity at constant pressure and density:
where λ is the thermal conductivity of the substance, W/(cm °C); α is the coefficient of thermal conductivity, cm2/s; Cp is the specific heat capacity at constant pressure, J/(g °C); and ρ is the bulk density, g/cm3.
The measurement of the thermal conductivity of solidified glasses is a complex experimental problem. The most popular experimental method for measuring thermal conductivity is the laser flash method. The measured quantity in the laser flash method is the temperature conductivity, and the thermal conductivity is calculated by formula (1), which requires the determination of other parameters in separate experiments. Direct methods for measuring thermal conductivity include, for example, the heated wire method, which is used to measure the thermal conductivity of molten salts [1]. However, this method may not be applicable to studying real radioactive samples of APS and BSS containing HLW.
The specialists of Mayak Production Association have accumulated extensive experience in determining the thermal stability and specific heat capacity of APS and BSS using the DSC method.
DSC measures the heat flux related to changes in the structure of materials as a function of time and temperature in a controlled atmosphere, accompanied by the absorption or release of heat.
Thermal stability is one of the main indicators of the quality of a phosphate glassy compound. According to NP-019-2015, the permissible values include the preservation of properties, including uniformity, strength, and water resistance, when exposed to the temperatures created during storage of the compound, including due to the heat released from the compound. As a result of the long-term storage of vitrified HLW at elevated temperatures, structural changes in the matrix are possible, leading to its devitrification and crystallization.
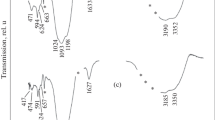
An example of the results of a study of the thermal stability of APS by the DSC method is shown in Fig. 1. The process of violations of thermal stability starts to become noticeable in its properties only in the region of the softening temperature (or glass transition), i.e., at about 425°C. At temperatures up to 400°C, APS with the included radionuclides remains X-ray amorphous, regardless of the time it is kept at this temperature. Starting from 581°C and above, crystallization of phosphate glass occurs, which intensifies with time when it is stored at elevated temperatures. The crystallization peak has a maximum at a temperature of 610°C.
According to NP-019-2015, when storing a glassy compound, the limiting temperature should be 100°C lower than the glass transition temperature.
Thus, it is of practical interest to determine the thermophysical characteristics (in particular, the thermal conductivity coefficient) of various types of APSs and BSSs containing HLW in the temperature range up to 325°C. However, the thermal conductivity of glasses was not determined by DSC until recently at Mayak Production Association.
For the first time, a practical procedure for studying thermal conductivity by DSC was described in 1985 by Hakvoort and Van Reijen [2], who proposed monitoring the melting of a pure metal located on the upper surface of a cylinder or disk made of the studied material. During the heating of such a system, the melting point of the metal is reached in the measuring cell of the DSC analyzer, and its temperature remains constant until all the metal is melted. Thus, the temperature of the upper surface of the disk at this moment is constant and known. The temperature of the lower surface of the disk and the heat flux supplied to it are measured by a DSC analyzer. It was proposed to calculate the thermal conductivity of the sample from the known temperature difference between the two surfaces of the disk and the heat flux.
The advantage of DSC measurements is the ability to measure the specific heat of a material using the same device and calculate the thermal conductivity using formula (1), which makes it possible to obtain the entire range of thermophysical characteristics of glassy matrices. The method for determining the heat capacity consists of comparing the heat fluxes of the baseline, the analyzed, and the standard sample.
THEORY
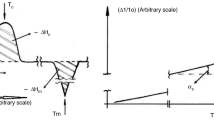
The appearance of a DSC signal on the example of metal melting is clearly illustrated by the diagram shown in Fig. 2.
Based on this diagram, before the start of melting (t1), the sample and reference temperatures are the same. During melting (t1 – tm), the temperature of the sample does not change. The sample receives heat. The temperature of the reference sample continues to rise. The temperature difference increases until the complete melting of the metal (tm). The sample temperature then takes on the value of the reference temperature (t2). The temperature difference is reduced. From value (t2) the temperatures of the sample and reference are again the same. The area of peak A is proportional to the heat of fusion.
Next, we consider the theoretical and methodological issues of determining the thermal conductivity by the DSC method.
The layout of the test sample on the sensor of the DSC analyzer is shown in Fig. 3.
Layout of the test sample: h is the height of the studied cylindrical sample; Tm is the temperature of the molten pure metal; TG is the reference sensor temperature; φ is the measured heat flux from the sensor to the sample without a crucible and to the powder in the crucible; Ts is the temperature of the sensor (the temperature of the lower surface of the cylindrical sample).
In stationary conditions, the heat flux φ, W, through a sample with thermal resistance is proportional to the temperature difference between the lower and upper boundaries of the sample and is calculated by the formula
where Rs is the thermal resistance of the sample, °C/W and ∆T is the temperature difference at the sample boundary, °C.
The thermal resistance of the sample is determined by the thermal conductivity of the material and the geometry of the sample and is calculated by the formula
where λ is the thermal conductivity of the sample, W/(m °C); A is the cross-sectional area of the sample, m2; and h is the height of the studied APS or BSS sample, m.
The value of the heat flux from the analyzer sensor to the metal on the upper surface of the sample depends not only on the thermal resistance of the sample itself but also on the thermal resistance of the sensor–sample boundaries (R1) and sample–metal (R2). Therefore, formula (2) should be rewritten in the form
where Ts is the temperature of the sensor under the sample, °C; Tm is the temperature of molten pure metal, °C; R1 is the thermal resistance of the boundaries of the sensor–crucible–sample, °C/W; and R2 is the thermal resistance of the sample–crucible–metal interfaces, °C/W.
In the experiments, for the reproducibility of values R1 and R2, the gaps at the boundaries were set to the minimum. Under these conditions, we can assume that when using samples with the same cross section, values R1 and R2 do not depend on the sample, and we can introduce parameter Rt:
Parameter Rs and the desired coefficient of thermal conductivity of sample λ can be determined provided that the quantities included in Eqs. (4) and (5), φ, Rt, Ts, and Tm, are known. Since pure metal is used, the value Tm during melting is known. Values φ and Ts are determined by the DSC analyzer in the course of measurements, and the value of Rt can be found from a series of measurements. If Rt \( \ll \) Rs, this quantity can be neglected. In this case, to determine the thermal conductivity coefficient λ it is sufficient to take just one melting curve.
Substituting Eq. (3) into expression (2), we obtain the formula
Formula (6) is valid only during the melting of pure metal. In this case ΔT in formula (6) is numerically equal to the temperature difference Ts at any time t and the melting point of the metal.
where \({{\varphi }_{t}}\) is the heat flux at time t, W; \({{\varphi }_{{{\text{onset}}}}}\) is the heat flux at the point where the metal melts, W; \({{T}_{{{\text{onset}}}}}\) is the temperature when the metal starts melting, °C; and S is the slope coefficient, the tangent of the slope of the DSC curve, W/°C (see Fig. 4).
From Eqs. (4)–(7), we obtain the equation
Having carried out two measurements (one with a sample in the crucible and metal, and the second with an empty crucible and metal), we can calculate the value of the thermal conductivity coefficient λ, taking into account the thermal resistances Rt
where \(\Delta h\) is the sample height, m; S1 is the slope of the DSC curve with the standard metal, W/°C; S2 is the slope of the DSC curve for the sample and metal, W/°C; \({{T}_{{{\text{2max}}}}}\) is the temperature at any time t2, taken on the melting curve of the metal in an aluminum crucible when measuring a sample in a crucible, °C; \({{T}_{{{\text{1max}}}}}\) is the temperature at any time t1, taken from the metal melting curve in an aluminum crucible when measuring an empty crucible without a sample, °C; and Rt is the thermal resistance, which is found from a series of measurements with samples with known thermophysical characteristics (the table value of the thermal conductivity of the material), °C/W.
Assuming the constancy of the contact thermal resistances included in Rt in the transition from the standard samples to samples from the studied material makes it possible to determine the thermal conductivity coefficient for APS and BSS.
The error of this method ranges from ±5 to ±10% [2].
EXPERIMENTAL
The core of our experiments to determine the thermal conductivity of APS and BSS is as follows. To measure the thermal conductivity according to the method described above, standard samples of pure metal with a known melting point were used: indium, tin and lead, melting at temperatures of 156.6, 231.9, and 327.5°C, respectively.
The measurements were performed on a STA F3 Jupiter synchronous thermal analysis instrument. This device was not chosen by chance, since at Mayak Processing Association, it was made by special order in a design that allows placing the measuring cell of the device in a glove box for working with active samples separately from the main control unit and electronics (Fig. 5).
An empty ceramic crucible with a volume of 90 µL is used as the reference sample for the measurement. Unlike the original version of the method [2], when pure metal was placed on a cylindrical sample (in our case, glass), we used an aluminum crucible with a volume of 20 μL without a lid. A sample (about 80 mg) of pure metal was placed in the crucible in such a way that the metal completely covered the bottom of the crucible during melting. The crucible thus prepared can be used several times. In addition, after two experiments, it was checked that the melting temperature and enthalpy of melting of the metal in the crucible did not change, because any deviation from the original values means either the formation of an alloy (possibly the appearance of a second peak) or oxidation. For this purpose, a separate experiment was carried out without glass. The studies were carried out in an argon atmosphere with a heating rate of 10°C/min.
In order to take the thermal resistances Rt into account using formula (10) and obtain the proportionality coefficient, a series of experiments were preliminarily carried out with the reference samples. A similar approach to the calculation of the proportionality coefficient was used by specialists from Lobachevsky Nizhny Novgorod State University when measuring the thermal conductivity of tellurite glasses by the DSC method [3]. The coefficients of proportionality obtained in their studies made it possible to obtain the values of the thermal conductivity of the studied samples with an error of not more than 4%.
In our experiments, Teflon (fluoroplast-4) samples with a known value of thermal conductivity \({{\lambda }_{{{\text{table}}}}}\) (0.25 W/m K), which are convenient for sample preparation of samples of various heights, were used as the reference samples. The objects of study in this work were inactive samples of APS and BSS, whose composition is given in Table 1. These glasses were synthesized by specialists from the central plant laboratory of the Federal State Unitary Enterprise PA Mayak in 2021 and correspond to the compositions of melts used or planned to be used for the immobilization of HLW.
Figure 6 shows a photograph of the sample preparation table, where the studied glasses, Teflon samples (fluoroplast-4), and ceramic and aluminum crucibles with the standard samples are visible.
The obtained experimental DSC diagrams for determining the slope S are shown in Figs. 7–9.
The thermal conductivity coefficient is calculated by formula (9) according to the calculated values of the slope coefficients. To assess the reliability of the obtained values, the results of studies of measuring the thermal conductivity of glasses of similar composition using other methods were analyzed.
In 2019, specialists from Mayak Production Association, together with the Institute of High-Temperature Electrochemistry, Ural Branch of the Russian Academy of Sciences, measured the thermal conductivity of APS and BSS samples by the method of coaxial cylinders in the temperature range from 300 to 1150°C [4]. In this paper, we studied changes in the glass transition temperature range and above depending on the composition of glasses and melts.
The temperature dependence of the thermal conductivity coefficient of glasses with a composition similar to our samples is shown in Fig. 10.
The experimental values of the thermal conductivity coefficient for APS and BSS by the DSC method, obtained in our work by processing the DSC diagrams in Figs. 7–9 and the thermal conductivity values at high temperatures, published in [4], are given in Table 2. The linear dependence, adjusted by four points with the known temperature for determining the thermal conductivity, is shown in Fig. 11. The deviation from linear behavior of the temperature dependence of the thermal conductivity coefficient for APS at temperatures of about 400°C can be explained by a violation of the thermal resistance of glass due to the approach to the softening phase. For BSS, this phase begins at temperatures 50–70°C higher than for APS.
Then we proceeded to determine the heat capacity. As noted above, the determination of the heat capacity using DSC consists of comparing the heat fluxes of the analyzed and calibration samples (in our experiments, sapphire was used as the standard). The error of this method is 2.5%.
All the experiments were performed using the same program:
— setting the initial temperature to 40°C,
— isothermal exposure for 20 min,
— heating to a temperature of 450°C,
— isothermal exposure for 20 min,
— cooling to room temperature.
The heat capacity of the samples, Cp,samples, J/(kg °C), is calculated by the formula
where \({{Q}_{{{\text{sample}}}}},\) \({{Q}_{{{\text{standard}}}}},\) and \({{Q}_{{{\text{empty cruc}}}}}\) are the heat fluxes of the sample, standard, and empty crucible, respectively, J; msample and \(~{{m}_{{{\text{standard}}}}}\) are the mass of the sample and standard, respectively, kg; \({{C}_{{p,\,{\kern 1pt} {\text{standard}}}}}\) is the heat capacity of the standard (sapphire), J/(kg °C).
The results of determining the heat capacity are shown in Fig. 12.
It should be noted that using DSC measurements, the heat capacity of a sample is determined over the entire temperature range, and the thermal conductivity in the proposed method was determined for several exact temperatures equal to the melting temperatures of the standard samples, from which, if necessary, the curve of change in thermal conductivity versus temperature is plotted.
The results of determining the thermophysical characteristics of inactive samples of APS and BSS of various compositions are presented in Table 3.
CONCLUSIONS
In this study, we tested the method of the experimental determination of the thermal conductivity of inactive APSs and BSSs by the DSC method.
The obtained experimental values of the thermal conductivity coefficient and specific heat capacity of inactive APSs and BSSs are consistent with the characteristics of glasses of similar composition published in the scientific publications.
The main advantages of DSC measurements of thermal conductivity are as follows: measurement of the specific heat capacity of a material on the same device and calculation of thermal conductivity, which allows obtaining the entire range of thermophysical characteristics; and small sample sizes, which is an important criterion for subsequent work with highly active samples to create a database on the characteristics of accumulated and newly formed APSs and BSSs.
The disadvantages of this method include complex sample preparation, which can affect the error in the results obtained. To reduce the measurement error of thermal conductivity and achieve the best reproducibility of thermal resistance, it is recommended to fill the air gaps between the sensor and the sample and the sample and the crucible with heat-conducting oil; and the cylindrical sample under study should have a diameter equal to the diameter of the bottom of the aluminum crucible with pure metal.
Therefore, the proposed method can be used to determine the thermophysical characteristics of real samples of vitrified HLW of any composition.
The results obtained during the experiments can be further used to make proposals on the target quality indicators of the glassy compound used in the technology of conditioning liquid HLW.
REFERENCES
Smirnov, M.V., Khokhlov, V.A., and Filatov, E.S., Thermal conductivity of molten alkali halides and their mixtures, Electrochim. Acta, 1987, vol. 32, no. 7, pp. 1019–1026.
Hakvoort, G. and van Reijen, L.L., Measurement of the conductivity of solid substances by DSC, Thermochim. Acta, 1985, no. 93, pp. 317–320.
Plekhovich, A.D., Kut’in, A.M., and Dorofeev, V.V., Measurement of thermal conductivity of (TeO2)0.72(WO3)0.24(La2O3)0.04 glass by DSC, Vestn. Nizhegor. Univ. im. N.I. Lobachevskogo, 2012, no. 5 (1), pp. 99–102.
Remizov, M.B. and Kozlov, P.V., Thermal and electrical conductivity of melts of aluminophosphate and borosilicate glasses containing simulators of high-level waste from SNF processing, Fiz. Khim. Stekla, 2019, vol. 45, no. 2, p. 120.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kazakov, V.A., Starovoitov, N.P., Dudkin, V.A. et al. Study of the Thermophysical Properties of Aluminophosphate and Borosilicate Glasses by DSC. Glass Phys Chem 49, 564–572 (2023). https://doi.org/10.1134/S1087659623600552
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659623600552