Abstract
The bioactive glasses 60SiO2·(36 – x)CaO·4P2O5·xZnO (x = 1, 3, 5 mol %) were prepared by the green synthesis based on the hydrothermal reaction of starting precursors at 160°C during 24 h. The uniqueness of this method is that it did not use any acid or base catalysts. Several physical-chemical methods such as BET, XRD, and SEM analysis were used for characterization of the synthetic glasses before and after in vitro experiment in SBF solution. The obtained result shows that the synthetic glasses 60SiO2·(36 – x)CaO·4P2O5·xZnO are amorphous materials and have mesoporous structures with pore size in range of 5.5–21.4 nm. The ZnO addition acted as an agent to change the properties of glass materials. The obtained bioactivity of synthetic glasses followed the order: sample x = 1 > sample x = 3 > sample x = 5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Over the past 50 years, bioactive glasses (BGs) have been used as artificial materials for repairing damaged and diseased bone due to their ability to connect to natural bone through the formation of mineral hydroxyapatite (HA) layer [1]. Structurally, bioactive glass materials consist of oxides like SiO2, Na2O, CaO, P2O5, B2O3, MgO, ZnO that are interconnected together to make an inorganic polymer with non-cyclic structure [1].
Bioactive glasses can be synthesized by two main processes, including the melting and sol-gel methods [2]. Recently, the sol-gel method is frequently used since it offers several advantages over the traditional melting method. The sol-gel method can synthesize bioactive glasses at low temperatures, preventing the loss of final products because of P2O5 volatilization at high temperatures. Furthermore, the sol-gel method can synthesize bioactive glasses with porous structure and high value of specific surface area, which enhances the bioactivity of synthetic bioactive glasse within the in vitro and in vivo experiments [3, 4].
When bioactive glasses are attached to bone tissue, bioactivity needs to be controlled to determine how long the artificial graft can bond with natural bone. Biologically beneficial elements such as Mg, Sr, Al, Cu, Fe, Ag, and Zn are frequently added to the composition of sol-gel synthetic glasses to regulate their bioactivity [5–11]. Among them, Zn plays an important role in bone formation due to its ability to inhibit the activity of osteoclasts and increase the differentiation of osteoblasts [12, 13].
Depending on the Zn content as well as the composition of the synthesized system, the bioactive glasses exhibit different properties. A. Balamurugan et al., was synthesized the bioactive glass 64SiO2·26CaO·5P2O5·5ZnO (mol %) by the sol-gel method. The authors showed that the combination of ZnO not only reduced the bioactivity but also increased osteoblast growth [6]. A.M. El-Kady et al., was also successfully fabricated 58SiO2·(33 – x)CaO·9P2O5·xZnO (x = 1, 3, 5 wt %) by sol-gel method. All synthesized bioactive glasses showed porous structure, high surface area, and good bioactivity in simulated body fluid (SBF) [14].
The results obtained by J. Bejarano et al., showed that the content of doped ZnO affected the structure, bioactivity, and biocompatibility of two bioactive glass systems 60SiO2·36CaO·4P2O5 (mol %) and 60SiO2·25CaO·11Na2O·4P2O5 (mol %). Especially, the increase in the content of doped ZnO reduced the formation of apatite minerals on the surface of the glasses after in vitro experiments [15].
As mentioned, many ZnO-doped bioactive glasses have been synthesized by the sol-gel method. In the sol-gel synthesis, the catalyst (acid or alkaline) is introduced in very small quantities and often not used [5], or nitric acid is used [6, 9]. Therefore, using axit or alkaline as a catalyst in bioactive synthesis is harmful to the environment and especially reduces the potential applications of bioactive glass such as biomedical applications. The ZnO-doped bioactive glasses synthesized by the “green chemistry method” without acid/base catalysts are of great significant not only in their use as artificial bone materials, but also as additives in toothpaste or cosmetics. Green chemistry method is the utilization of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products [16]. In previous work, we used the acid-free hydrothermal method to synthesize a three-component glass system SiO2·CaO·P2O5 (mol %) [16]. The mixture of initial precursors was heat-treated in a hydrothermal reaction system at 160°C for 24 h. The gel product was dried and then sintered at 700°C for 3 h. The synthesized glass has an amorphous structure and exhibits good bioactivity within in vitro experiment.
In this work, we firstly synthesized three-component glasses SiO2–CaO–P2O5 doped with ZnO at the ratios of 1, 3 and 5 (mol %) by the green synthesis without using acid or base catalysts based on the hydrothermal reaction. The phase composition, structural morphology, and bioactivity of bioactive glasses were studied and evaluated.
EXPERIMENTAL
Synthesis of Bioactive Glasses
Bioactive glasses 60SiO2·(36 – x)CaO·4P2O5·xZnO (x = 1, 3, 5 mol %) were prepared by green synthesis without using acid or base catalysts. The composition of bioactive glasses are selected according to the previous bioactive glasses which were synthesized by using sol-gel method [14, 15]. The compositions of bioactive glasses are presented in Table 1. The main regents used for bioactive glass synthesis are tetraethyl orthosilicate (TEOS, Sigma Aldrich), triethyl phosphate (TEP, Sigma Aldrich), calcium nitrate tetrahydrate (Ca(NO3)2·4H2O, Merck), and zinc nitrate hexahydrate (Zn(NO3)2·6H2O, Merck). Firstly, the initial precursors were added successively at 30 min intervals to the reaction vessel containing distilled water under continuously stirring condition. The molar ratio of H2O/TEOS was surveyed and chosen to be 60. Then, the reaction mixture was transferred to a hydrothermal system and heated at 160°C for 24 h. The resulting gel was dried at 100°C for 12 h and calcined at 700°C for 3 h to achieve the synthetic glasses.
In vitro Experiment
In vitro experiments were carried out according to the protocol of Kokubo et al. [17]. The samples of bioactive glasses 60SiO2·(36 – x)CaO·4P2O5·xZnO (x = 1, 3, 5 mol %) were immersed in SBF (Simulated Body Fluid) solution at 37°C for several times. Inorganic composition of synthetic SBF is similar to that of blood in the human body. The bioactivity of glasses was proved through the formation of hydroxyapatite (HA) layer as function of soaking times.
Characterization
Textural property of synthetic glasses was investigated by N2 adsorption/desorption measurement on a Quantachrome Instruments Quadrasorb, SI. The types of porous structure were determined based on the feature of adsorption/desorption isotherms according to the IUPAC classification [18, 19]. The values of specific surface area were obtained by multi-point BET (Brunauer Emmett Teller) measurements in a range of relative pressure from 0 to 0.3. The pore size distributions were achieved by BJH (Barrett Joyner Halenda) calculation from the desorption curves. The phase composition of synthetic glasses was verified by X-ray diffraction (XRD) on a D8- Advance with a step size of 0.02° in the range of 10°–70° (2θ). The surface morphology of synthetic glasses was investigated by Scanning Electron Microscopy (SEM) observation on SEM Hitachi S-4800.
RESULTS AND DISCUSSION
Textural Analysis
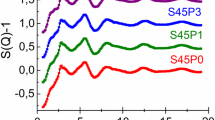
The textural properties of glasses 60SiO2·(36 – x)CaO·4P2O5·xZnO (x = 1, 3, 5 mol %) were investigated by the N2 adsorption/desorption analysis. The isotherm and pore size curves are presented in Fig. 1 and the textural values are reported in Table 2. According to IUPAC classification, the isotherms of all glass samples (x = 1, 3, 5) are type IV, characteristic of mesoporous materials with pore sizes in range of 2 to 50 nm [19]. The distributions of pore size are measured by BJH method using the desorption isotherm branch. The pore sizes of synthetic glasses range from 5.5 to 21.4 nm depending on their compositions. The obtained values of specific surface area and pore size show the irregular variation according to the addition of ZnO content. The specific surface area decreased when the ZnO content increased from 1 to 3 mol %; however, it increased sharply when the added ZnO content was 5 mol %. This is explained by the dependence of the specific surface area not only on the pore size but also on the number of pores present in the synthetic material. The best value of specific surface area is obtained for sample x = 5 (108.8 m2/g). This result is consistent with the previous study, where the best value of specific surface area was achieved for synthetic sample with lower CaO/SiO2 ratio [20].
Phase Identification
Figure 2 depicts the XRD diagrams of synthetic glasses 60SiO2·(36 – x)CaO·4P2O5·xZnO (x = 1, 3, 5 mol %). In the sample x = 1, the XRD pattern presents a halo diffraction with a center of about 25° (2θ), which is indicated the amorphous characteristic of synthetic glass [14–16]. When amount of added ZnO increased from 1 to 5, the shapes of the diffraction patterns were slightly changed, but still showed wide diffraction halos without any crystalline peaks. Taken together, these results confirm that the ZnO-doped glasses are amorphous materials.
Morphological Observation
The morphology of synthetic bioactive glasses 60SiO2·(36 – x)CaO·4P2O5·xZnO (x = 1, 3, 5 mol %) are represented in SEM images (Fig. 3). Samples were measured at different magnifications of 30 000, and 50 000 times. As shown in Fig. 3, the morphology of obtained glasses is changed when increasing the content of doped ZnO. The sample x = 1 shows a fairly uniform surface structure consisting of quasi-spherical particles connected together to make a regular-porous structure of the synthetic material. When adding 3 mol % of ZnO to the glass system (sample x = 3), the quasi-spherical particles seem to disappear and are replaced by aggregates of larger particles that combine to produce a more porous structure than sample x = 1. This observation is consistent with the above textural analysis, where the sample x = 3 shows the decrease of specific surface area and the increase of pore size compared to the sample x = 1. The surface structure of synthetic glass changed strongly as the doped-ZnO content increase from x = 3 to x = 5 mol %. Particularly, the sample x = 5 exhibits a completely different structure in comparison with the sample x = 3, which shows an uneven structure which consists of distinct porous regions, making the high value of specific surface area as the above analysis.
Bioactivity Investigation
Figures 4 and 5 provide the XRD diagrams of 60SiO2·(36 – x)CaO·4P2O5·xZnO (x = 1, 3, 5 mol %) after immersion in SBF solution for 3 and 5 days, respectively. Noted that the diffraction patterns of the samples after 1 day of immersion did not show much change in their features, so they are not included here. The XRD pattern of hydroxyapatite (HA) is used to verify the bioactivity of glass samples after in vitro experiments [JCPDS no. 09-0432]. After 3 days of immersion in SBF solution, all synthetic samples appeared crystalline peaks which are characteristic for the HA phase (Fig. 4). This observation confirms the bioactivity of synthetic glasses. When the content of ZnO in the glass composition was 1 (sample x = 1), there was the appearance of two characteristic peaks of HA at about 26° and 32° (2θ), which are corresponding to the reflection planes (002) and (211), respectively. Meanwhile, at x = 3, and x = 5, accompanying glasses just showed one characteristic peak of HA at 32° (211) and the peak intensities of these glasses were much lower than those of glasses at x = 1. These results demonstrated that the bioactivity of synthetic glass is obviously reduced when the content of added ZnO increased from 1 to 5 mol %. On the other hand, after 5 days of in vitro experiment, the XRD patterns of sample x = 1 showed most of the characteristic peaks for HA phase, while those of samples x = 3 and x = 5 exhibited only 3 peaks (Fig. 5). Moreover, the peak intensities of sample x = 3, and x = 5 were lower than that of sample x = 1. Therefore, with 1 (mol %) of doped ZnO, the synthetic glass 60SiO2·(36 – x)CaO· 4P2O5·xZnO (x = 1) showed good bioactivity. From above analysis, the bioactivities of synthetic glasses 60SiO2·(36 – x)CaO·4P2O5·xZnO (x = 1, 3, 5 mol %) can be arranged in the following order sample x = 1 > sample x = 3 > sample x = 5. These obtained results was consistent with the previous studies [15, 20], confirmed the effect of ZnO addition on decreasing the bioactivity of synthetic glasses. Together these results provide important insight into the role of ZnO in the controlling of bioactivity of synthetic bioactive glasses.
Figure 6 presents the SEM images of synthetic glasses 60SiO2·(36 – x)CaO·4P2O5·xZnO (x = 1, 3, 5 mol %) after 3 and 5 days in SBF solution. After in vitro experiment, the glass samples changed their surface morphology in comparison to the initial samples in Fig. 3. These differences can be explained in part by the interaction between the glass surface and SBF solution. The glass sample x = 1 showed clearly the appearance of well-defined small crystals on their surfaces after 5 days of in vitro experiments, whereas samples x = 3 and x = 5 did not clearly show the formation of new crystalline phase. The obtained results were in full agreement with XRD results with the conclusion about the decrease of bioactivity when the ZnO content added into glass system increased from 1 to 5 (mol %).
CONCLUSION
The bioactive glasses 60SiO2·(36 – x)CaO· 4P2O5·xZnO (x = 1, 3, 5 mol %) were prepared by the green synthesis based on the hydrothermal reaction. The well-mixed precursors were introduced into the hydrothermal system and heated at 160°C for 24 h. The resulting gels were dried and calcined at 700°C to achieve the vitreous systems. Textural analysis confirms the mesoporous structures of all synthetic glasses. The best value of specific surface area was obtained for the sample x = 5 with lower CaO/SiO2 ratio. Phase investigation mentions amorphous characteristic of synthetic glasses. In vitro experiment highlights the effect of ZnO addition on the bioactivity of synthetic glasses. The bioactivity of glasses is arranged in the following order: sample x = 1 > sample x = 3 > sample x = 5. In summary, the green synthesis can replace the conventional sol-gel method for the synthesis of ZnO-doped bioactive glasses with their interesting properties. The green synthesis without using of any acid or base catalysts brings the benefits to the environment, researchers, and offers potential application in bone regeneration.
REFERENCES
Hench, L.L., The story of bioglass, J. Mater. Sci. Mater. Med., 2006, vol. 17, pp. 967–978.
Jones, J.R., Review of bioactive glass: From Hench to hybrids, Acta Biomater., 2013, vol. 9, pp. 4457–4486.
Owens, G.J., Singh, R.K., Foroutan, F., Alquaysi, M., Han, C.M., Mahapatra, C., Kim, H.W., and Knowles, J.C., Sol-gel based materials for biomedical applications, Prog. Mater. Sci., 2016, vol. 77, pp. 1–79.
Zheng, K. and Boccaccini, A.R., Sol-gel processing of bioactive glass nanoparticles: A review, Adv. Colloid Interface Sci., 2017, vol. 249, pp. 363–373.
Sharifianjazia, F., Parvina, N., and Tahriri, M., Synthesis and characteristics of sol-gel bioactive SiO2–P2O5–CaO–Ag2O glasses, J. Non-Cryst. Solids, 2017, vol. 476, pp. 108–113.
Balamurugan, A., Balamurugan, A., Balossier, G., Kannan, S., Michel, J., Rebelo, A.H., and Ferreira, J.M., Development and in vitro characterization of sol-gel derived CaO–P2O5–SiO2–ZnO bioglass, Acta Biomater., 2007, vol. 3, pp. 255–262.
Salman, S., Salama, S., and Mosallam, H.A., The role of strontium and potassium on crystallization and bioactivity of Na2O–CaO–P2O5–SiO2 glasses, Ceram. Int., 2012, vol. 38, pp. 55–63.
Kheshen, A.E., Khaliafa, F.A., Saad, E.A., and Elwan, R.L., Effect of Al2O3 addition on bioactivity, thermal and mechanical properties of some bioactive glasses, Ceram. Int., 2008, vol. 34, pp. 1667–1673.
Errol, M., Özyuguran, A., and Çelebican, O., Synthesis, characterization, and in vitro bioactivity of sol-gel-derived Zn, Mg, and Zn–Mg co-doped bioactive glasses, Chem. Eng. Technol., 2010, vol. 33, pp. 1066–1074; Proceedings, 2020, vol. 62, no. 6, p. 12.
Bari, A., Bloise, N., Fiorilli, S., and Novajra, G., Vallet-Regi, M., Bruni, G., Torres-Pardo, A., González-Calbet, J.M., Visai, L., and Vitale-Brovarone, C., Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration, Acta Biomater., 2017, vol. 55, pp. 493–504.
Baino, F., Fiume, E., Miola, M., Leone, F., Onida, B., and Verné, E., Fe-doped bioactive glass-derived scaffolds produced by sol-gel foaming, Mater. Lett., 2019, vol. 235, pp. 207–211.
Hadley, K.B., Newman, S.M., and Hunt, J.R., Dietary zinc reduces osteoclast resorption activities and increases markers of osteoblast differentiation, matrix maturation, and mineralization in the long bones of growing rats, J. Nutr. Biochem., 2010, vol. 21, pp. 297–303.
Popp, J.R., Love, B.J., and Goldstein, A.S., Effect of soluble zinc on differentiation of osteoprogenitor cells, J. Biomed. Mater. Res., Part A, 2007, vol. 81, pp. 66–769.
E-Kady, A.E. and Ali, A.F., Fabrication and characterization of ZnO modified bioactive glass nanoparticles, Ceram. Int., 2012, vol. 38, pp. 1195–1204.
Bejarano, J., Caviedes, P., and Palza, H., Sol-gel synthesis and in vitro bioactivity of copper and zinc-doped silicate bioactive glasses and glass-ceramics, Biomed. Mater., 2015, vol. 10, 025001.
Ta Anh Tuan, Guseva, E.V., Nguyen Anh Tien, Ho Tan Dat, and Bui Xuan Vuong, Simple and acid-free hydrothermal synthesis of bioactive glass 58SiO2· 33CaO·9P2O5 (wt %), Crystals, 2021, vol. 11, pp. 283-1–12.
Kokubo, T. and Takadama, H., How useful is SBF in predicting in vivo bone bioactivity, Biomaterials, 2006, vol. 27, no. 15, pp. 2907–2915.
Thommes, M., Physical adsorption characterization of nanoporous materials, Chem. Ing. Technol., 2010, vol. 82, pp. 1059–1073.
Thommes, M., Kaneko, K., Neimark, A.V., Olivier, J.P., Rodriguez-Reinoso, F., Rouquerol, J., and Sing, K.S.W., Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution, Pure Appl. Chem., 2015, vol. 87, nos. 9–10, pp. 1–19.
Atkinson, I., Anghel, E.M., Predoana, L., Mocioiu, O.C., Jecu, L., Raut, I., Munteanu, C., Culita, D., and Zaharescu, M., Influence of ZnO addition on the structural, in vitro behaviour and antimicrobial activity of sol-gel derived CaO–P2O5–SiO2 bioactive glasses, Ceram. Int., 2016, vol. 42, no. 2, pp. 3033–3045.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
The authors contributed equally (co-first authors)
Rights and permissions
About this article
Cite this article
Bui Thi Hoa, Phuc, L.H., Hien, N.Q. et al. Structure, Morphology and Bioactivity of Bioactive Glasses SiO2–CaO–P2O5 Doped with ZnO Synthesized by Green Synthesis. Glass Phys Chem 48, 273–279 (2022). https://doi.org/10.1134/S1087659622040058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659622040058