Abstract
Hydrosilicates of the kaolinite group (Al2Si2O5(OH)4 · nH2O) with spherical, nanotubular, and platy particle shapes are synthesized in hydrothermal conditions. The morphology and size of the particles, as well as the porous-textural characteristics, are studied by scanning electron microscopy (SEM) and low-temperature nitrogen absorption. The sorption capacity of the samples relative to the cationic and anionic dyes is investigated on the example of methylene blue and carmoisine. A comparative analysis is conducted for the characteristics of synthetic aluminosilicates and their natural analogs: nanotubular halloysite and kaolinite with platy particles. It is shown that characteristics such as porosity (diameter and volume of pores) and the specific surface area (from 11 m2/g for platy to 470 m2/g for spherical particles) can differ among kaolinite subgroup aluminosilicates having a different morphology. It is shown that the synthetic aluminosilicate with spherical particles (dav = 300 nm) is an effective universal sorbent of differently charged ions from aqueous solutions and its characteristics are better than for structural analogs and synthetic aluminosilicates of a different morphology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Constantly growing production scales and increasing the requirements on the quality of water are stimulating the search for more efficient methods of removing pollution from natural and waste water. Various organic substances, hard particles, heavy metal ions, and dyes are related to harmful and hazardous pollutants for which many traditional cleaning methods are ineffective [1–3]. Adsorption is a globally recognized method for treating polluted water due to its efficiency and ease of applicability. Some adsorbents, for example, activated carbon, resins, mesoporous hybrid xerogel, clays, and polymer composites were investigated for the removal of water-soluble organic dyes such as methylene blue, rhodamine, and methyl violet [4–9]. Adsorbents such as activated carbon and resins, which are widely used for domestic and waste water treatment are characterized by a relatively low adsorption capacity, high cost of regeneration, and pollution of sorption colons. The search for the best alternative adsorbents is currently ongoing.
In the past few decades, directions connected with obtaining new materials using nature-like technologies have been intensively developed. Directed hydrothermal synthesis can be related to these technologies. This method makes it possible to obtain various nanomaterials with the given characteristics, such as morphology, chemical composition, particle size, surface properties, and characteristics of the porous space [10–12]. Synthesis of porous aluminosilicates under the conditions of directed hydrothermal synthesis offers opportunities for the development of a new generation of efficient adsorbents.
Porous hydroaluminosilicates, particularly those related to the kaolinite group, are attracting significant scientific attention. The minerals of this group are characterized by a two-layered structure containing the layers formed by the octahedral and tetrahedral networks. Such hydroaluminosilicates have an Al2Si2O5(OH)4 · nH2O general formula, where n = 0 (typical for kaolinite, nacrite, and dickite) and n = 2 (typical for hydrated species of halloysite) [13]. The elevated interest in the minerals of the kaolinite subgroup is caused by its large variety of particle morphology: tubes, nanospheres, fibers, cylinders, etc. [14]. For kaolinite particles, the platy morphology is more typical, although in some cases, the formation of spherical particles is possible [13]. Despite their similar chemical composition, the chemical properties of these materials can differ significantly. It was shown in [15] that halloysite has a higher absorption capacity regarding cationic (rhodamine) and anionic (chromazurol) dyes compared to kaolinite. Apparently, this is connected with the different compositions of halloysite tubes on the internal and external surfaces of the layer: negatively charged SiO2 and positively charged Al(OH)3 (according to the authors of [14]), which allows it to adsorb differently charged ions efficiently.
To date, insufficient attention has been paid to the effect of the morphology of particles of the kaolinite subgroup on the formation of the adsorption and texture properties. The use of directed hydrothermal synthesis makes it possible to obtain hydroaluminosilicates of the kaolinite subgroup with a defined morphology and to study their properties, primarily, sorption on the example of cationic and anionic dyes: methylene blue (MB) and carmoisine (C).
EXPERIMENTAL
Synthesis of samples. The samples were synthesized under hydrothermal conditions in steel autoclaves with Teflon and platinum crucibles of 40 and 60 mL, respectively; the filling coefficient was 0.8. The synthesis was carried out in an aqueous medium using the drying hydrogels of the corresponding compositions as the starting reagents. The initial gels were prepared using Tetraethoxysilane TEOS ((C2H5O)4Si, special purity grade), Al(NO3)3 ⋅ 9H2O (reagent grade), HNO3 (reagent grade, 65 wt %), NH4OH (special purity grade), and ethanol. The gels obtained were dried at a temperature of 100°C for 24 h and then calcined at 550°C for 2 h. Hydrothermal treatment of the gels was conducted at temperatures ranging from 200 to 350°C and the synthesis time was 24–96 h. The products of the crystallization were rinsed with distilled water and dried at 100°C for 12 h.
The morphology, specific surface area, and sorption characteristics of the synthesized samples were compared with natural kaolinite samples (NevaReaktiv, KBE-2) and tubular halloysite (Halloysite nanoclay, Sigma-Aldrich, Product of Applied Minerals, United States).
Sorption of dyes. To study the sorption properties of synthesized samples, the following reagents were used: methylene blue C16H18N3SCl (reagent grade, ZAO Vekton), activated carbon (DARCO®, Fluka, M = 12.01 g/mol, analytical grade), and carmoisine С20H12N2Na2O7S2. The sample (20 mg) was dispersed in an aqueous dye solution with a concentration of 0.1 g/L. The experiments were conducted with stirring and regular sampling for 120 min, which corresponded to the time when equilibrium was established. Each sample was filtered, and the dye concentration in the filtrate was determined as the arithmetic mean of three measurements.
The dye concentration was determined by absorption UV spectroscopy using a LEKISS2109UV spectrophotometer according to the optical density at a wavelength of 245 (for MB) and 515 (for C) nm.
The sorbent’s capacity (amount of adsorbed substance, mg/g) was determined by formula (1):
where Ci is the initial concentration of a dye solution (g/L), Cf is the final concentration after sorption (g/L), Vsol is the volume of the dye solution (L), and mads is the mass of the sorbent’s sample (g).
The dye solution having the initial concentration was prepared by the weight method. The concentration was calculated by the formula
where q is the dye’s weight, g and V is the solution’s volume (deionized water), L.
Methods of investigation. X-ray diffraction analysis was performed using a D8-Advance (Bruker) powder diffractometer with CuKα radiation in the following mode: 40 kV/40 mA, Vantec-1 position-sensitive detector, θ–θ geometry, and measurement range of 2θ = 5°–70° (step of 2θ = 0.0224°).
The porous structure was studied by low-temperature nitrogen adsorption using a NOVA 1200e (Quantachrome, United States) device. Deaeration was studied at 300°C for 12 h. The Brunauer–Emmett–Taylor (BET) method was applied to determine the specific surface area. The pore distribution by size was determined using the Barrett-Joyner-Halenda (BJH) method for the desorption branch of the isotherm. Calculations were performed using the NOVAWin-2.1 software.
The particles’ morphology was studied by scanning electron microscopy (SEM) using a Merlin (Carl Zeiss) device with a field emission cathode, a GEMINI-II column of electronic optics, and an oil-free vacuum system. The beam current and accelerating voltage were 2 nA and 21 kV, respectively. The powders were put onto a conducting carbon adhesive tape without additional treatment.
RESULTS AND DISCUSSION
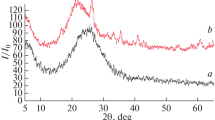
A comparison of the characteristic peaks of the synthesized samples with natural forms (Fig. 1) confirms the formation of aluminosilicates of the kaolinite subgroup.
The analysis of the data of electron microscopy (Fig. 2) and XRD analysis allows making conclusions about the formation conditions of aluminosilicates of the kaolinite group having a certain morphology. At the hydrothermal treatment at temperatures below 200°C, an amorphous compound is formed (Fig. 1, spectrum a). Low-temperature treatment at temperatures ranging from 200 to 220°C promotes the formation of spherical particles with the average diameter of 300 nm by the SEM data (Fig. 2a).
When the heat treatment is conducted in the temperature range of 300 to 350°C, kaolinite samples with tubular (the average length and external diameter are 1500 and 300 nm, respectively) and platy (the average length and thickness are 1200 and 200 nm, respectively) particle morphology can be obtained (Figs. 1, spectra d and e, 2b, 2c). In the temperature range of 300 to 350°С, samples with mixed morphology were obtained but increasing the temperature led to the domination of the platy morphology. A comparison of the SEM images of the synthesized samples with a tubular morphology and natural halloysite showed the similarity of the morphology and particle size.
Figure 3 presents the results of the sample investigation by low-temperature nitrogen adsorption. Similar shapes of hysteresis loops are typical for samples having nanotubular (both natural and synthetic) and platy morphologies. This shape can be considered as the 4th isotherm type according to the IUPAC classification (Figs. 3a, 3c, 3d), which indicates the existence of both micro- and mesopores. The hysteresis loop can be attributed to the H3 type. It is believed that this type of isotherm indicates the presence of aggregates of platy particles forming slit-shaped pores. The shape of the hysteresis loop for the synthesized aluminosilicate with a spherical morphology of the particles is significantly different from the samples having a different morphology (Fig. 3b). This loop can be attributed to the 4th isotherm type and the hysteresis loop is typical for the H2 type. It is assumed that the H2 type indicates mesoporous structures with different pore shapes. The Table 1 lists the values of the specific surface area and volume and diameter of the pores of the studied samples.
As follows from the data in the Table 1, the specific surface area of the sample with the spherical morphology of the particles significantly differs from the other samples. Its value is 470 m2/g, which is almost ten times higher than that for natural nanotubular halloysite (41 m2/g) and about 40 times higher than for natural kaolinite (11.4 m2/g). The specific surface area of the synthetic aluminosilicates of a different morphology is smaller and its values lie in the range between the specific surface areas characteristic for natural halloysite and kaolinite. Aluminosilicates of the spherical morphology have the largest pore volume, and the average pore diameter is in the same range as for the other samples (3.5–4 nm). The only exception is a sample of natural halloysite having the average pore size of 11 nm.
Figure 4 demonstrates the results of the study of the sorption capacities of samples and their comparison with activated carbon. As is seen from Fig. 4a, the sorption capacity of natural halloysite relative to the MB cationic dye is 40.5 mg/g. The sorption capacity of the samples having a spherical morphology is 2.5 times higher than that for natural halloysite and it is comparable with the values typical for activated carbon (100 mg/g). For the activated carbon sample, sorption equilibrium is reached only after 2 h of continuous stirring, while the aluminosilicate sample with spherical particles completely absorbs the dye for 30 min. The values of the sorption capacity relative to MB for the samples with tubular and platy morphology are significantly lower compared to the other samples (19 and 8.5 mg/g, respectively), which is close to the sorption capacity of natural kaolinite (15 mg/g). Figure 4b presents the sorption isotherms for C (anionic dye). The samples of activated carbon and aluminosilicate with a spherical morphology showed high values of the sorption capacity relative to the cationic dye (100 mg/g) while the sorption capacity of natural halloysite decreased significantly (to 8 mg/g). Samples with platy and tubular particle morphology and the natural kaolinite sample demonstrated a lower sorption capacity than C: 6.7 and 6.5 mg/g, respectively. This indicates that there is almost no absorption of the dye. The drop of the sorption capacity of the samples relative to the anionic dye can be explained by the charge of their surface. A negative surface charge is typical for aluminosilicates in the studied range of pH values. Anionic dye absorption is ineffective.
The sorption capacity of nanotubular halloysite and synthetic aluminosilicate samples with tubular morphology relative to C is very low (lower than 8 mg/g). In some works (for example, 14–16]), it was assumed that the features of a nanotubular halloysite structure, namely, the existence of the negative and positive changes on the external and internal surfaces, respectively, allow it to efficiently absorb differently charged ions. As is seen from the presented results, this assumption is not always true. Nanotubular halloysite can effectively sorb from solutions only positively charged ions, and the sorption of negatively charged ions is insufficient. A similar result was obtained in [17] where the adsorption process was studied for the negatively charged ions of a 5-fluorouracil anticancer drug in solutions by porous aluminosilicates with a different morphology. The inefficiency of the use of nanotubular halloysite forms was shown in comparison with other aluminosilicates (zeolites and layered silicates of the smectite group).
The samples of synthetic aluminosilicates with spherical particle morphology showed their universality, and they can be used as an efficient sorbent of both negatively and positively charged ions from solutions. Apparently, such sorption behavior is related to the large specific surface area and features of the pore space of such a morphology because the direct dependence of the adsorption capacity of a number of enterosorbents on their specific surface area was shown earlier [18].
CONCLUSIONS
A comparative analysis of the textural and sorption properties of synthetic aluminosilicate samples of the 1 : 1 kind having a different morphology and their natural analogs (nanotubular halloysite and kaolinite with platy particles) was conducted. It was shown that synthetic aluminosilicate with a spherical particle shape has a high specific surface area, which is ten times higher than for the samples of a different morphology) and the high values of the sorption capacity relative to the MB cationic dye (100 mg/g) and C anionic dye (to 100 mg/g). The sorption characteristics of the synthesized aluminosilicate are superior not only to the structural analogs and synthetic aluminosilicates with a different morphology but also to activated carbon, which is widely used for waste and industrial water treatment. Aluminosilicate samples with a nanotubular and platy morphology are not particularly efficient in the sorption of both positively and negatively charged ions from solutions. Based on the obtained results, it can be concluded that the synthesized hydroaluminosilicate with spherical particles is a highly effective universal sorbent of differently charged ions from aqueous solutions, which has great prospects for practical application as a sorbent for solving the special problems of environmental protection.
REFERENCES
Akpor, O.B., Ohiobor, G.O., and Olaolu, T.D., Heavy metal pollutants in wastewater effluents: Sources, effects and remediation, Adv. Biosci. Bioeng., 2014, vol. 2, no. 4, pp. 37–43.
Chawla, S., Uppal, H., Yadav, M., Bahadur, N., and Singh, N., Zinc peroxide nanomaterial as an adsorbent for removal of Congo red dye from waste water, Ecotoxicol. Environ. Saf., 2017, vol. 135, pp. 68–74.
Khin, M.M., Nair, A.S., Babu, V.J., Murugan, R., and Ramakrishna, S., A review on nanomaterials for environmental remediation, Energy Environ. Sci., 2012, vol. 5, no. 8, pp. 8075–8109.
Liu, F., Chung, S., Oh, G., and Seo, T.S., Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal, ACS Appl. Mater. Interfaces, 2012, vol. 4, pp. 922–927.
Hai, Y., Li, X., Wu, H., Zhao, S., Deligeer, W., and Asuha, S., Modification of acid-activated kaolinite with TiO2 and its use for the removal of azo dyes, Appl. Clay Sci., 2015, vol. 114, pp. 558–567.
Zhang, Q., Yan, Z., Ouyang, J., Zhang, Y., Yang, H., and Chen, D., Chemically modified kaolinite nanolayers for the removal of organic pollutants, Appl. Clay Sci., 2018, vol. 157, pp. 283–290.
Yang, R., Li, D., Li, A., and Yang, H., Adsorption properties and mechanisms of palygorskite for removal of various ionic dyes from water, Appl. Clay Sci., 2018, vol. 151, pp. 20–28.
Gouthaman, A., Azarudeen, R.S., Gnanaprakasam, A., Sivakumar, V.M., and Thirumarimurugan, M., Polymeric nanocomposites for the removal of acid red 52 dye from aqueous solutions: Synthesis, characterization, kinetic and isotherm studies, Ecotoxicol. Environ. Saf., 2018, vol. 160, pp. 42–51.
Golubeva, O.Yu. and Pavlova, S.V., Adsorption of methylene blue from aqueous solutions by synthetic montmorillonites of different compositions, Glass Phys. Chem., 2016, vol. 42, no. 2, pp. 207–213.
Golubeva, O.Yu., Effect of synthesis conditions on hydrothermal crystallization, textural characteristics and morphology of aluminum-magnesium montmorillonite, Microporous Mesoporous Mater., 2016, vol. 224, pp. 271–276.
Mascolo, G., Synthesis of anionic clays by hydrothermal crystallization of amorphous precursors, Appl. Clay Sci., 1995, vol. 10, nos. 1–2, pp. 21–30.
Korytkova, E.N., Pivovarova, L.P., Semenova, O.E., Drozdova, I.A., Povinich, V.F., and Gusarov, V.V., Hydrothermal synthesis of nanotubular Mg–Fe hydrosilicate, Russ. J. Inorg. Chem., 2007, vol. 52, no. 3, pp. 338–344.
Peng, Y., Thill, A., and Bergaya, F., Nanosized Tubular Clay Minerals: Halloysite and Imogolite, Amsterdam: Elsevier, 2016.
Joussein, E., Petit, S., Churchman, J., Theng, B., Righi, D., and Delvaux, B., Halloysite clay minerals—a review, Clay Miner., 2005, vol. 40, no. 04, pp. 383–426.
Zhao, Y., Abdullayev, E., Vasiliev, A., and Lvov, Y., Halloysite nanotubule clay for efficient water purification, J. Colloid Interface Sci., 2013, vol. 406, pp. 121–129.
Lvov, Y., Wang, W., Zhang, L., and Fakhrullin, R., Halloysite clay nanotubes for loading and sustained release of functional compounds, Adv. Mater., 2015, vol. 28, no. 6, pp. 1227–1250.
Golubeva, O.Yu., Alikina, Yu.A., Brazovskaya, E.Yu., and Ugolkov, V.L., Peculiarities of the 5-fluorouracil adsorption on porous aluminosilicates with different morphologies, Appl. Clay Sci., 2020, vol. 184, 105 401.
Filippova, V.A., Lysenkova, A.V., Ignatenko, V.A., and Dovnar, A.K., The comparative description of the adsorption properties of enterosorbents, Probl. Zdorov. Ekol., 2016, vol. 47, no. 1, pp. 41–46.
Funding
This work was financially supported by the Russian Foundation for Basic Research (project no. 19-33-90089).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by N. Saetova
Rights and permissions
About this article
Cite this article
Alikina, Y.A., Kalashnikova, T.A. & Golubeva, O.Y. Sorption Capacity of Synthetic Aluminosilicates of the Kaolinite Group of Various Morphology. Glass Phys Chem 47, 42–48 (2021). https://doi.org/10.1134/S1087659621010028
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659621010028