Abstract
The research of the hydrolytic polycondensation kinetics of tetraethoxysilane (TEOS) is required in order to understand the sol-gel synthesis processes and identify the factors determining the direction of these processes. An extensive analysis of these problems permits us to predict appropriate ways for the synthesis of new substances with the predetermined properties. Studying the kinetics of the polycondensation of TEOS above the gel point will allow solving problems of the gels’ strength and the gels’ degree of structuring and thus optimizing the conditions of the further processing materials for the production of the final synthesis products. In this paper the results of the research on the structuring kinetics of the silica in the modeled aqueous-alcoholic solutions of TEOS, including boron-containing solutions, before and after the gel point at various molar ratios of H2O : SiO2 and pH values of 2.0 and 6.0 by the differential kinetic spectrophotometry are presented. The kinetic scheme developed by the authors and the mathematical tool allow determining the degree of the direction of the process of silica polycondensation. The data obtained on the model systems were applied to the description of the silica structuring process in the acid solutions resulting from the treatment of the single-phased sodium borosilicate glass. The obtained results will form the base for the interpretation of the experimental data on the kinetics of the silica structuring contained in the unstable phase of the two-phased alkali-borosilicate glass during the leaching process; i.e., it will allow predicting the dissolution rate, forms of existance, gelation time, strata formation time and, as a consequence, the formation of some porous structure of the resulting porous glass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Classical concepts of the process of the hydrolytic polycondensation of alkoxysilanes, including tetraethoxysilane (TEOS), and the methods used for investigating various stages of this process expounded in Brinker’s and Scherer’s monograph [1].
The research methods for the state of silica in the solutions can be divided on the structural (physical), the classic analytic, and the physico-chemical (kinetic) methods.
The structural research methods (29Si-NMR [2–13]; PMR [13, 14–16]; IR-spectroscopy [17], including with the Fourier conversion [18]; gas and gas-liquid chromatography [10, 14, 19–23]); small-angle X-ray scattering (SAXS) [24, 27]) allow defining associates of silica with a large molecular weight, as well as determining the stereoisomers and the functional groups (OH−, OR−, CH3O−, etc.) connected to the silicon–oxygen tetrahedrons.
A specific feature of the nuclear magnetic resonance is the absence of the requirement for the sample’s purity. Research can be done on the liquid solutions as well as the solids. The method permits identifying a wide range of the molecular forms of silica beginning with the monomer [10, 12, 13, 25] and calculating the gelation time on the change in the quantity of water in the reaction system [26]. As well as all the enumerated further instrumental research methods, NMR requires complex instrumentation and is characterized by a sufficiently large value of the relative standard deviation of 2–10%.
The application of the small-angle X-ray scattering is limited by the systems with an inhomogeneous structure. It allows estimating the dependence between the scattering angle and the radiation intensity that permits defining the inhomogeneous elements with a larger mass than a trimer’s. From the concentration of each oligomer at any moment in time we can obtain information not only on the rate and the polycondensation mechanism but also data for forecasting the characteristics of the obtained material [27]. The results obtained by this method are of special value for planning the condition of the organic and non-organic synthesis for both scientific and industrial purposes.
Gas chromatography (GC) is characterized by a high velocity, possibility of determining impurities and studying small quantities (from 0.01 to 50 µL of the liquid). It was shown that it was possible to divide the contributions of oligomeries that are different by mass and isomery in the application of research on the polycondensation process and the silica structuring using the capillary gas chromatography [19].
Raman scattering allows defining the polymer’s structure according to the number of bonds whose number is proportional to the particle size [28]. The method is applied in the spectral range where there is no noticeable absorption by the sample. There is a laborious sample preparation process that includes separation of the liquid phase from the suspended solid as the latter enhances scattering. Solids are studied by a surface-reflected laser beam. In both cases the perfect impurity removal is necessary. Despite the technological difficulties, the main advantage of Raman spectroscopy is the possibility to observe the growth of polymer particles both before and after the gelation point.
The kinetic parameters of the fluidity variation of the system are defined using acoustic and rheological methods [29, 30]. There are papers on the kinetics of structuring silicon alkoxides by dynamic light scattering [31–33], the photochemical technique [34], and high-temperature calorimetry [35].
The classic analytical methods allow determining only the total content of silica in the sample. Independent of the final method of determining silica (gravimetric, photometric, titrimetric, or spectral), the preliminary procedures of sample preparation must be performed. They involve reducing and dissolving the particles, transforming the silica into the monomeric form, and separating or masking the interfering components and, in some cases, the preliminary concentration [36–38].
The kinetic variants of methods for determining silica in the form of β-silicon-molybdenum heteropoly acid (HPA) using spectrophotometry method can conventionally attribute to the physic-chemical (kinetic) methods. The created variants allow identifying the monomers, dimers, trimers, olygomers (more complex low-molecular associates), and polymers (high-molecular forms) [39–41]; calculating the rate constants of hydrolysis and complexing [39, 42]; and dividing nominally polymer silicic acids into α, β, and γ-forms [43].
In this paper we reviewed the physicochemical base of our elaborated differential kinetic spectrophotometry for condensing systems based on TEOS. The obtained results were used to study the kinetics of structuring silica during the leaching process (by the acid treatment) of single-phase alkali-borosilicate (ABS) glass of 16.8Na2O · 39.0B2O3 · 44.2SiO2 (mol %), which corresponds to the composition of the unstable phase of two-phased ABS glass. The obtained results were used to study the process of gelation of “secondary silica” the in the porous glass pore space during the leaching process of the two-phase ABS glass of 7.6Na2O · 20.4B2O3 · 71.9SiO2 · 0.1Al2O3 (mol %).
Physicochemical principles of the differential kinetic (DK) spectrophotometry of condensing systems. DK-spectrophotometry is based on the classic absorption spectroscopy, which in contrast to the structural methods of analysis is fairly simple both instrumentally and experimentally. Currently the application of the method is well known in the analysis of food samples [44] and pesticides [45], in pharmacology [46, 47], and in determining different rhutenium associates [48], as well as the quantitative content of chemical analogs in two-component mixtures without prior separation [49, 50].
The fundamental distinction of our elaborated modification of DK-spectrophotometry from the techniques mentioned above is that the method is applied to analyze dynamical systems—hydroalcoholic TEOS solutions in which the hydrolytic polycondensation is taking place. Our method is based on the classic indicator reaction of silica in the form of a saturated complex of silicomolibdic heteropolyanion (HPA) of Keggin’s structure (molybdate method):
Silicomolibdic heteropolyanion (HPA) solutions are colored bright yellow and they remain in place when crystallization occurs. The complex may subsist in two isomeric forms: the cis-isomer is named the α-form and the trans-isomer is named the β-form [52–55].
The β-form irreversibly transforms into the α-form in the solutions [56]. The process of isomeric transformation can be slowed by the introduction of a 10-fold excess of the molybdate containing reagent (usually it is molybdic acid, H2MoO4, or its ammonium and sodium salts, (NH4)2MoO4 and Na2MoO4, respectively), creating a pH of 1.0 to 2.5 and by isolation the reaction vessel from light. The time required for the half-reaction for the isomeric transition reaction under these conditions is about 25 hours [38].
The extinction coefficient of the β-form is more than double the corresponding value for the α-form [52]. Since the time stability of the β-form in the solutions is sufficient for the spectrophotometric measurement, usually the time stability is used as the analytical form for determining the silica.
The kinetics of the silicon-molybdenum HPA were studied by photocalorimetry in the 1950s [57] and later by others [40]. The reaction of the monosilicic acid and the oligomeric forms of the silicic acids i.e. associates having more than one silicon atom in the silicon-oxygen chain and capable of depolymerization with the molybdate reagent is spent entirely [53, 58], which ensures the fixation of all low-molecular forms of the hydrolyzed silicon alkoxide in the compound named above. The possibility of the direct formation of HPA from the silicic acid gel [59] allows applying the molybdate reaction in studying the hydrolytic polycondensation of the alkoxides [60–63].
Based on the literature, we can conclude that formation of the β-form of silicomolibdic HPA from the silicon alkoxides, as well as TEOS, is possible only after the hydrolysis reaction has run its course. In this case the equation of the complexing reaction is
From the kinetic point of view, this process may be considered as the consecutive reaction of the pseudofirst order since the experiment was performed with an excess of the ammonium molybdate in the concentration range where the rate of the reaction does not depend on the reagent containing molybdate [61, 62].
The performed studies had shown that DK-spectrophotometry allows identifying the following molecular forms (associates) of silica, formed during the hydrolytic polycondensation of TEOS:
(1) Non-hydrated forms (R) are associates containing Si−O−R bonds (R is alkyl). Since the kinetics of this bond rupture do not depend on the size of the associate, the non-hydrated forms are assumed to be monomer molecules of alkoxysilane. This means that the structural unit reacting with molybdate accepts the Si−O−R bond; the quantity of these bonds is referred to one silicon atom. The consideration of the monomer molecule allows constructing balanced equations and determining the quantitative relationships between associates in silicon gram atom units. In other words that the dissociation energy in the silicon–oxygen tetrahedron does not depend on the substituents in the other three positions. Consequently, the average field approximation is accepted: the considered molecular forms are interpreted as averaged. This means, for example, that four particles Si(OH)3(OR) can be equivalently presented as three particles Si(OH)4 and one particle Si(OR)4.
The selection of these forms is related to the possibility of the direct estimation of their impact to the total kinetics process. Taking this into consideration, these forms are interpreted as molecular forms of the corresponding silicic acids.
(2) The low-molecular forms include monomers (M), dimers (D), and trimers (Tr). The separation of these forms is associated with the possibility of directly evaluating their contribution to the kinetics of the “molybdate” reaction. With allowance made for the above mentioned, these forms are treated as molecular forms of the corresponding silicic acids.
(3) The macromolecular weakly structured forms, olygomers (Ol), are associates which degenerate under the influence of ammonium molybdate with the formation of the silicomolybdic HPA.
(4) Macromolecular strongly structured forms, polymers (P), are associates which does not degenerate under the influence of the molybdate reagent with the formation of the silicomolybdic HPA.
For the qualitative analysis of the kinetics of the β‑silicomolybdic HPA formation, we propose a kinetic scheme based on the fact that molybdate ions interact only with the monomer form (i.e., Si−OH bonds) and adding a reagent consisting of molybdate entirely suppresses all processes of the association of the silica molecular forms and that the interaction time of silica associates with molybdate is roughly proportional to the size of the associates [55, 57]; thus, the formation of β-silicomolybdic HPA is possible only through the intermediate formation of monomers, the Si(OH)4 particles; the remaining particles must be transformed into the monomer form for the possibility of the molybdate reaction. Thus, the kinetic form of the entire process may be presented as the multistep reaction involving the steps of the hydrolysis of non-hydrated forms, the dissociation of weakly structured associates, and the interaction of molybdate ions with the Si−OH bonds:
where β is the β-silicomolybdic acid and the values noted under the arrows are the rate constants of the corresponding steps. Based on the above points, it is clear that these values are the kinetically averaged characteristics.
During the preliminary experiments, we calculated the rate constants for the reaction of the β-silicomolybdic HPA formation in the solutions containing non-polymerized TEOS and determined the kinetic coefficient for the hydrolysis [42]. The obtained value for TEOS kM = 2.77 min–1 coincides with the value 2.6 min–1 obtained in [39] and kH = 1.00 min–1 at рН 1 coincides with the value from [1]. Based on the Levenberg–Marquardt algorithm the mathematical method of experimental data processing that we have elaborated is explained in detail in [64].
The proposed kinetic scheme was validated by comparing the data on the distribution of the molecular forms of silica during the hydrolytic polycondensation of TEOS with the results of capillary gas chromatography under similar conditions [19].
EXPERIMENTAL TECHNIQUE
First, 1.2 mL of 4 M HCl solution are collected in a 50 mL calibrated flask, after that 2 mL of 10% ammonium molybdate (NH4)2MoO4 and 1 mL of ethyl alcohol are added; then the distilled water is added to the mid-point of the volume. After that the obtained solution is mixed thoroughly in order to avoid a concentration gradient appearing. Volume (or weight) aliquot of a studied solution containing not more than 4.46 × 10–4 mol/mL of silica is added to the obtained reaction mixture and the starting time of the complex formation is recorded. After that the solution is mixed and increased to the mark by distilled water. After remixing the solution we perform the photometric measurements in a 1cm quartz cell using the UNICO 2800 spectrophotometer with a temperature-controlled Peltier cell at wavelength λ = 390 nm relative to the mix of reagents as the blank reagent. The change in the transmission density is measured at 30 sec intervals until the limiting optical density, which remains at a constant value for at least two hours, which is enough for performing the spectrophotometric experiment and is in a good agreement with the stability time of inorganic solutions containing silicomolybdic HPA. The set of data points obtained in this way (minimum of 10) is sufficient for the construction of the kinetic curves based on the experimental data and required calculations. Using the mix of reagents as a blank solution allows reducing the possibility of an error variable related to the purity of the reagents used and offsets the background absorption of the reagent containing molybdate. The spectrophotometric measurement error is 0.2%, which fits into the error interval of the technique used [65]. The pH values were recorded using a рН-meter Sartorius PP-15.
RESULTS AND DISCUSSION
Again note that the associates’ stability is considered only in relation to the “molybdate” reaction.
Kinetics of the Hydrolytic Polycondensation in Hydroalcoholic TEOS Solutions Before and After the Gelation Point
The pH of media and the H2O : Si molar ratio are considered as variable characteristics of the process during the study of the kinetics of the hydrolytic polycondensation of TEOS before and after the gel point by the molybdate method. The process was performed at pH 2.0 and 6.0 (the values correspond to the maximum and minimum gel time in acidic media for silicon alkoxides) at molar ratio of H2O : Si = 4, 6, 13. Using the hydrochloric acid when performing the acid-catalyzed hydrolysis is due to the fact that it is one of the widely accepted catalytic materials and its density in the solution is close to the density of the other components of the reaction mixture in contrast to other acids (for example, H2SO4).
The initial stages of the process are studied for two hours; and after the gelation point, the process is studied for 10 days.
Comparing the data obtained at various pH values shows that the inversion of the complexing rate at the pH varying from 2.0 (catalyzed polycondensation) to 6.0 (non-catalyzed polycondensation) is observed in all cases. With the catalyzed polycondensation reaction, the complexing rate is reduced, which means that the catalyzed polycondensation runs with the stepwise growth of the associates’ size. For noncatalyzed polycondensation (рН 6.0), the situation is the opposite: after rapid polycondensation for 5 minutes, the weakly structured silica is transformed into finer associates during the studied time frame. This is due to the increasing role of the reverse reactions, hydrolysis and alcoholysis, which prevents the growth of the polymer chain and leads to the decay of associates and the appearance of less stable formations.
In the case of the catalyzed condensation of TEOS, the growth of the associates is slower the higher the dilution. This is due to the reduction in the concentration of TEOS; therefore, the hydrolysis and condensation rates slow down, which contributes to the appearance of the counter reactions even within the conditions of the acid catalysis. In the case of uncatalyzed polycondensation, the molar ratio of H2O : Si did not significantly affect the speed of the process. In the case of noncatalyzed polycondensation (pH 6.0) the polymer formation (up to 20%) is observed only in TEOS solutions at a stoichiometric ratio of Si : H2O = 1 : 4. A negligible (1–2%) formation of polymers is found in TEOS solutions at Si : H2O = 1:6. At Si : H2O = 1 : 13 no polymers are formed in the studied time interval. A noticeable formation of stable associates in TEOS begins in 45 min after hydrolytic polycondensation starts. In the case of catalyzed polycondensation (pH 2.0), stable growth of the number of polymers is observed. The stronger the dilution the greater the growth.
The decrease in the molybdate reaction rate is related to the growth of stability relative to the reaction conditions of the gel network and the addition of low-molecular associates to the gel network that is observed in all the studied model systems after the gel point at pH 6.0 in all dilutions. The presence of monomers and dimers of silica proves the validity of the conclusion of the classical Flory–Stockmayer theory on the possibility of the presence of low-molecular silica associates in condensing systems after the gel point [66]. At the same time, the low-molecular forms or even unstable olygomers of silica was not observed in the studied systems.
Kinetics of Hydrolytic Polycondensation in Boron-Containing Aqueous-Alcoholic Solutions of TEOS before and after the Gelation Point
The addition of boric acid in the standard TEOS solution does not affect the kinetics of the “molybdate” reaction [42]. It allows us to investigate the impact of borate ions on the kinetics of the condensation by directly comparing the kinetic data on solutions with boron acid additions and without them.
The experimental data show that the addition of boric acid inhibits the “molybdate” reaction at pH 6.8 and catalyzes this reaction at pH 2.0. At pH 6.0 the reaction rate thus increases in the following order of values of B : Si–0.25 and 0.5 for H2O : Si = 4 : 0. For ratios of H2O : Si = 6 and 13, the maximum inhibition of the molybdate reaction occurs at B : Si = 0.25. At pH 2.0 the effect is the opposite. This may be due to the fact that during the increase of the boron concentration and reduction of the pH the boron coordination relative to oxygen varies from 3 to 4. The increase of the boron content in the system leads to the formation of stable low-molecular associates containing B—O—Si bonds. This results in the distension of the silicon-oxygen network of the gel with the formation of weakly structured oligomer forms.
The calculations show that the addition of boric acid results in a decrease in the proportion of monomers with an increase in the proportion of oligomers. It confirms the formation of stable low-molecular borosilicate associates with the following distension of the gel matrix and the formation of weakly structured oligomer forms. Thus, the formation and growth of the gel with a growth of the boron content is accompanied by restructuring. An increase of the boron coordination leads to a change in the stability of borosilicate associates and the appearance of structures containing =B—O—B= bonds.
The discovered dependences open up space for the application of the proposed method to study borosilicate systems based on the division of the fractions impacts of silicon and boron.
Studying the Silica Structuring Process in the Acidic Solutions Obtained in the Treatment of Single-Phased Sodium Borosilicate Glass
The research objects were powders of single-phase sodium borosilicate glass with a stoichiometric composition (calculated as analyzed mol %) of 16.8Na2O · 39.0B2O3 · 44.2SiO2. A powder glass fraction with average particle size of 0.515 mm was obtained by passing through sieves of 0.63 and 0.4 mm. The glass was processed in 0.1 М HCl at 26°C with forced stirring (400 rpm). The initial relation between the surface area of the glass and solution volume S/V was 1 cm–1 when using the powder quantity of 1.0159 g of glass per 50 mL of the treating solution. The extraction kinetics of the glass components into the solution were studied for 1 hour. For estimation of the reproducibility of the obtained results, three replicated experiments were performed for each time period of acid treatment of the sodium borosilicate glass powder [41].
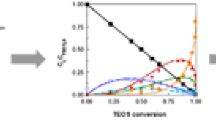
In the time range t ~ 15–20 min, silica continues to transform into a solution and is accompanied by the rearrangement by the molecular forms of the silica contained in the digested solution for a fraction of 0.515 mm (Fig. 1).
It was found that the monomer and oligomer forms of silica are the main products of the interaction of sodium borosilicate glass and a 0.1 M HCl solution. The relation discovered (in the long-term treatment of glass in an acid solution) between the formation of the structure of silica and the continuous increase in the pH of the solution is in accordance with the classic theory of the condensing SiO2 polymerization in the studied pH range. The calculations performed in the kinetic model allow obtaining information on the actual fractions of the molecular forms of silica at the given moment in time.
Based on the results presented in Fig. 1, it is seen that in the time range from 15 to 50 min the relation of the molecular forms of silica remains unchanged. After that the proportion of the oligomer molecular forms begins to increase with minimization of the content of the other forms, which corresponds to the experimental data presented in Fig. 2. This can be explained by the system reaching the state when the precipitation process of the secondary silica begins.
At the second stage, the aging of the solutions obtained in the acid treatment of the powders of single-phased sodium borosilicate glass was studied using the example of an 0.815-mm fraction. The selection of this fraction is explained by the possibility of covering a broader range of values of S/V, which is an important characteristic of the leaching process. Measurements were performed starting from the end of the acid treatment process and after defined periods of time until achieving a balance in the solutions hereinafter referred to as structured.
Its presence was determined by the consistency of the weakly structured silica content in the studied solutions. As a result, the dependence of τi, of the time of the reaction’s occurrence for the formation of β-silicomolybdic HPA (where i is the number of days since the beginning of the solution’s existence as a separate system) on the aging of the solution was found (Fig. 3).
The presence of these facts is explained by the polymerization processes (2)–(4) occurring in the solutions:
Polymerization into separated particles with the formation of the siloxane bonds [1]:
Polymerization of the boric anhydride hydrates occurring through the formation of an orthoborate ion:
Formation of boron-silica associates:
The result of these processes is the formation of silica associates incapable of depolymerization. The time required for the structural equilibrium is ~15 days. The structural equilibrium of the studied solutions lasts for at least six months, which corresponds with the data on 0.1 M HCl solutions with silica and does not depend on S/V. Note the time dependence of the occurrence of the β-silicomolybdic HPA formation reaction at S/V = 0.3 (Fig. 3). In contrast to other relation of S/V, in this case, the monotonic reduction of the time of the formation of the complex is observed, which implies the formation of polymer forms of silica without the interconversion of its weakly associated forms.
The calculation of the silica monomer fraction contained in solutions after the acid treatment of the 0.815 mm fraction showed that all the structured silica is in the monomer form.
CONCLUSIONS
Studying the kinetics of the structuring of silica in hydroalcoholic TEOS solutions before and after the gelation point, including boron-containing solutions, by DK-spectrophotometry revealed that in all the investigated systems the degree of the hydrolytic polycondensation’s direction can be predicted by the results obtained in the first two hours after the process starts. This allows accelerating the time of the selection of the optimal conditions for the synthesis of materials with the predetermined complex of properties. Apart from the applied relevance, the calculated kinetic parameters of the reactions occurring are of interest in the sphere of phenomenological chemical kinetics. Also the limitation of the elaborated DK-spectrophotometry technique caused by the time stability of the β-form of the silicomolybdic HPA was found.
The performed kinetic studies on the solutions obtained by the acid treatment of single-phase ABS glass showed that they are dynamic systems in which the structuring process of silica were observed. This process fully corresponds to the classical theory of the inorganic silica polymerization in acidic solutions [67].
The described kinetic method for determining the degree of silica structuring transformed from the model single-phase ABS glass into the treated solution allows investigating the behavior of the silica contained in the unstable phase of two-phase ABS glass during the leaching process, i.e., predicting its dissolution rate, form, gelation time, strata formation time, and thus the formation of the porous structure of the obtained PG (porous glass). Such a prediction is necessary to determine the physico-chemical features of the leaching process of two-phase ABS glass for optimizing the synthesis conditions for PG with the predetermined structure parameters caused by gelation of the secondary silica. The obtained data can be used to describe the leaching process and to determine the strata formation mechanism.
REFERENCES
Brinker, C.J. and Scherer G.W., Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, San Diego: Academic, 1990.
Engelhardt, H.G., Altenburg, W., Hoebbel, D., and Wieker, W., 29Si-NMR-Spektroskopie an Silicatlosungen. IV. Untersuchungen zur Kondensation der Monokieselsaure, Z. Anorg. Allg. Chem., 1977, vol. 428, pp. 43–52.
Pouxviel, J.C., Boilot, J.P., Beloeil, J.C., and Lallemand, J.Y., NMR study of the sol-gel polymerization, J. Non-Cryst. Solids, 1987, vol. 89, pp. 345–360.
Pouxviel, J.C. and Boilot, J.P., Kinetics study of the acidic catalyzed polymerization of tetraethoxysilane by 29Si-NMR, in Better Ceramics through Chemistry III, Brinker, C.J., Clark, D.E., and Ulrich, D.R., Mater. Res. Soc. Symp. Proc., 1986, vol. 121, p. 39.
Boonstra, A.H. and Baken, J.M.E., Relation between the activity and reactivity of a TEOS, ethanol and water mixture, J. Non-Cryst. Solids, 1990, vol. 122, pp. 171–182.
Van Beek, J.J., Seykens, D., Jansen, J.B.H., and Schuiling, R.D., Incipient polymerization of SiO2 in acid-catalyzed TMOS sol-gel systems with molar water/alkoxide ratio between 0.5 and 32, J. Non-Cryst. Solids, 1991, vol. 134, pp. 14–22.
Bernards, T.N.M., van Bommel, M.J., and Boonstra, A.H., Hydrolysis–condensation processes of the tetra-alkoxysilanes TPOS, TEOS and TMOS in some alcoholic solvents, J. Non-Cryst. Solids, 1991, vol. 134, pp. 1–13.
Brunet, F. and Cabane, B., Populations of oligomers in sol-gel condensation, J. Non-Cryst. Solids, 1993, vol. 163, pp. 211–216.
Damrau, U. and Marsmann, H.C., The hydrolysis of oligomer intermediates in the sol-gel process, J. Non-Cryst. Solids, 1994, vol. 168, pp. 42–48.
Chiba, J., Sugahara, Y., and Kuroda, K., Novel polysiloxane formation process from dimethyldiethoxysilane in the presence of oxalic acid, J. Sol-Gel Sci. Technol., 1994, vol. 2, pp. 153–156.
Dong, H., Lee, M., Thomas, R.D., Zhang, Z., Reidy, R.F., and Mueller, D.W., Methyltrimethoxysilane sol-gel polymerization in acidic ethanol solutions studied by 29Si-NMR spectroscopy, J. Sol-Gel Sci. Technol., 2003, vol. 28, pp. 5–14.
Oubaha, M., Dubois, M., Murphy, B., and Etienne, P., Structural characterisation of a sol-gel copolymer synthesised from aliphatic and aromatic alkoxysilanes using 29Si-NMR spectroscopy, J. Sol-Gel Sci. Technol., 2006, vol. 38, pp. 111–119.
Gunji, T., Kaburagi, H., Tsukada, S., and Abe, Y., Preparation, properties, and structure of polysiloxanes by acid-catalyzed controlled hydrolytic co-polycondensation of polymethyl(methoxy)siloxane and polymethoxysiloxane, J. Sol-Gel Sci. Technol., 2015, vol. 75, pp. 564–573.
Hui, Y., Zishang, D., Zhonghua, J., and Xiaoping, X., Sol-gel process kinetics for Si(OEt)4, J. Non-Cryst. Solids, 1989, vol. 112, pp. 449–453.
Kuo, C.-F.J. and Chen, J.-B., Study on the synthesis and application of silicone resin containing phenyl group, J. Sol-Gel Sci. Technol., 2015, vol. 76, pp. 66–73.
Perchacz, M., Beneš, H., Kobera, L., and Walterová, Z., Influence of sol-gel conditions on the final structure of silica-based precursors, J. Sol-Gel Sci. Technol., 2015, vol. 75, pp. 649–663.
Orel, B., Ješe, R., Vilčnik, A., and Štangar, U.L., Hydrolysis and solvolysis of methyltriethoxysilane catalyzed with HCl or trifluoroacetic acid: IR spectroscopic and surface energy studies, J. Sol-Gel Sci. Technol., 2005, vol. 34, pp. 251–265.
Jäglid, U. and Lindqvist, O., Reaction rates of the consecutive transesterification of teos with methoxide ions and distribution reaction rates of ethoxytrimethylsilane and methoxytrimethylsilane in alkaline alcohol solutions, J. Non-Cryst. Solids, 1993, vol. 163, pp. 81–89.
Klemperer, W.G., Mainz, V.V., Ramamurthi, S.D., and Rosenberg, F.S., Solid state multinuclear magnetic resonance study of the sol gel process using polysilicate precursors, in Better Ceramics Through Chemistry III, Mater. Res. Soc. Symp. Proc., 1988, vol. 121, p. 15.
Brinker, C.J., Keefer, K.D., Schaefer, D.W., Assink, R.A., Kay, B.D., and Ashley, C.S., Sol-gel transition in simple silicates II, J. Non-Cryst. Solids, 1984, vol. 63, pp. 45–59.
Klein, L.C. and Garvey, G.J., Kinetics of the sol/gel transition, J. Non-Cryst. Solids, 1980, vols. 38–39, pp. 45–50.
Blum, J.B. and Ryan, J.W., Gas chromatography study of the acid catalyzed hydrolysis of tetraethylorthosilicate [Si(OC2H5)4], J. Non-Cryst. Solids, 1986, vol. 81, pp. 221–226.
Chojnowski, J., Cypryk, M., Kazmierski, K., and Rozga, K., The reactivity of silanol intermediates in the hydrolytic polycondensation of tetraethoxysilane in asidic media, J. Non-Cryst. Solids, 1990, vol. 125, pp. 40–49.
Sacks, M.D. and Sheu, R.-S., Rheological properties of silica sol-gel materials, J. Non-Cryst. Solids, 1987, vol. 92, pp. 383–396.
Artaki, I., Bradley, M., Zerda, T.W., and Jonas, J., NMR and Raman study of the hydrolysis reaction in sol-gel processes, J. Phys. Chem., 1985, vol. 89, pp. 4399–4404.
Peeters, M.P.J., Bernards, T.N.M., and Van Bommel, M.J., 17O-NMR of sol-gel processes of TEOS and TMOS, J. Sol-Gel Sci. Technol., 1998, vol. 13, pp. 71–74.
Cogan, H.D. and Setterstrom, S.A., Properties of ethyl silicate, Chem. Eng. News., 1946, vol. 24, pp. 2499–2501.
Zerda, T.W., Bradley, M., and Jonas, J., Raman study of the sol to gel transformation under normal and high pressure, Mater. Lett., 1985, vol. 3, pp. 124–126.
Vogelsberger, W., Seidel, A., and Fuchs, R., Contribution to the determination of kinetic parameters of the sol-gel transformation by rheological measurements, J. Colloid Interface Sci., 2000, vol. 230, pp. 268–271.
Griesmar, P., Ponton, A., Serfaty, S., Senouci, B., and Warlus, S., Kinetic study of silicon alkoxides gelation by acoustic and rheology investigations, J. Non-Cryst. Solids, 2003, vol. 319, pp. 57–64.
Arroyo, R., Campero, A., and Rodriguez, R., Aggregation profiles of silica sols in sol-gel process, Mater. Lett., 1993, vol. 16, pp. 89–95.
Rodriguez, R. and Salinas, P., A study of the kinetics of gelation of silica particles induced by lead ions in alcoholic solution, Mater. Lett., 1997, vol. 30, pp. 73–77.
Unger, B., Hähnert, M., and Nitzsche, R., Aging of acid-catalyzed silica sol—a dynamic light scattering study, J. Sol-Gel Sci. Technol., 1998, vol. 13, pp. 81–84.
Smirnova, N.P., Kikteva, T.A., and Eremenko, A.M., Photochemical technique for studying sol-gel-xerogel transitions in silica, Theor. Exp. Chem., 1996, vol. 32, pp. 272–275.
Maniar, P.D., Navrotsky, A., Rabinovich, E.M., Ying, J.Y., and Benziger, J.B., Energetics and structure of sol-gel silicas, J. Non-Cryst. Solids, 1990, vol. 124, pp. 101–111.
Myshlyaeva, L.V. and Krasnoshchekov, V.V., Analiticheskaya khimiya kremniya (Silicon Analytical Chemistry), Moscow: Nauka, 1972.
Kalinina, N.E., Gileva, K.G., and Khomutova, E.G., Microanalysis of silicates: an investigation into the natural and technical mineral formation, Trudy VII soveshchaniya po eksperimental’noi i tekhnicheskoi mineralogii i petrografii (Proc. 7th Conf. on Experimental and Technical Mineralogy and Petrography), Moscow: Nauka, 1966, pp. 61–66.
Piryutko, M.M. and Shmidt, Ya.A., State of silicic acid in solution, and methods for its colorimetric determination, Bull. Acad. Sci. USSR,Chem. Sci., 1953, vol. 2, pp. 545–550.
Coudurier, M., Baudru, B., and Donnet, J.-B., Étude de la polycondensation de l’acide disilicique, Bull. Soc. Chim. Fr., 1971, vol. 9, pp. 3147–3165.
Bogdanova, V.I., The reaction between silicate and molybdate ions as a method for assessing the polymerization of silicas, Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim., 1970, no. 2 (4), pp. 82–87.
Rakhimova, O.V., Tsyganova, T.A., Antropova, T.V., and Kostyreva, T.G., Spectrophotometric determination of molecular forms of silica in solution during the leaching of alkali borosilicate glasses, Glass Phys. Chem., 2000, vol. 26, no. 3, pp. 303–306.
Rakhimov, V.I., Rakhimova, O.V., Semov, M.P., and Shil’nikova, M.A., Kinetics of the interaction of silicon alkoxides with ammonium molybdate, Dokl. Akad. Nauk, 1998, vol. 360, no. 2, pp. 220–223.
Egorova, E.N., Metody vydeleniya kremnevoi kisloty i analiticheskogo opredeleniya kremnezema (Methods for the Separation of Silicic Acid and Analytical Determination of Silica), Moscow: Akad. Nauk SSSR, 1959.
Ni, Y., Xiao, W., and Kokot, S., A differential kinetic spectrophotometric method for determination of three sulphanilamide artificial sweeteners with the aid of chemometrics, Food Chem., 2009, vol. 113, pp. 1339–1345.
Deng, N., Ni, Y., and Kokot, S., Differential kinetic spectrophotometric determination of methamidophos and fenitrothion in water and food samples by use of chemometrics, Chin. J. Chem., 2010. https://doi.org/10.1002/cjoc.201090087
Pu, G., Yu, X., Pu, G., and Xi, F., Differential kinetic spectrophotometric determination of chlorpromazine hydrochloride and promethazine hydrochloride by chemometric method, Chin. J. Chem., 2006, vol. 26, pp. 1364–1367.
Ghasemi, J., Seraji, H.R., Noroozi, M., Hashemi, M., and Jabbari, A., Differential kinetic spectrophotometric determinations of ascorbic acid and l-cysteine by partial least squares method, Anal. Lett., 2004, vol. 37, pp. 725–737.
Qiao, Y., Wang, B., and Wu, J., Simultaneous determination of different ruthenium species by rate differential kinetic spectrophotometry, J. Renewable Sustainable Energy, 2018. https://doi.org/10.1063/1.4996359
Nikitina, S.A., Demyanova, T.A., Stepanov, A.V., Lipovskii, A.A., and Nemtsova, M.A., Kinetic analysis of actinides using differential spectrophotometry, J. Radioanal. Nucl. Chem., 1979, vol. 51, pp. 393–399.
Bayanov, V.A., Rakhimov, V.I., Rakhimova, O.V., and Semov, M.P., Spectrophotometric differential kinetic method for the determination of germanium and silicon in the presence of each other in the GeO2–SiO2 systems, Glass Phys. Chem., 2016, vol. 42, no. 2, pp. 214–217.
Pope, M., Heteropoly and Isopoly Oxometalates, New York: Springer, 1983.
Strickland, J.D.H., Isomeric forms of silicomolybdic acid, Chem. Ind., 1950, vol. 20, pp. 392–404.
Strickland, J.D.H., The preparation and properties of silicomolybdic acid. I. The properties of alpha silicomolybdic acid, J. Am. Chem. Soc., 1952, vol. 74, pp. 862–867.
Strickland, J.D.H., The preparation and properties of silicomolybdic acid. II. The preparation and properties of β-silicomolybdic acid, J. Am. Chem. Soc., 1952, vol. 74, pp. 868–871.
Strickland, J.D.H., The preparation and properties of silicomolybdic acid. III. The preparation and properties of β-silicomolybdic acid, J. Am. Chem. Soc., 1952, vol. 74, pp. 872–876.
Jean, M., Transition of isomeric forms of silicomolybdic acid, Chim. Anal., 1955, vol. 37, pp. 125–131.
Alexander, G.B., The polymerization of monosilicic acid, J. Am. Chem. Soc., 1954, vol. 76, pp. 2094–2096.
Nikitina, E.A., Geteropolisoedineniya (Heteropoly Compounds), Moscow: Goskhimizdat, 1962.
Nikitina, E.A. and Prytkova, E.V., Equilibrium systems: Saturated heteropoly acids—organic solvents, Russ. J. Gen. Chem., 1959, vol. 30, pp. 1410–1417.
Hoebbel, V.D. and Wieker, W., Über Kondensationreaktionen der Monokieselsäure, Z. Anorg. Allgem. Chem., 1973, vol. 400, pp. 148–160.
Stade, H., Die Umsetzung von Monokieselsaure mit Molybdänsäure, Z. Anorg. Allgem. Chem., 1978, vol. 446, pp. 29–38.
Stade, H., Die Umsetzung von kondensierten Kieselsauren mit Molybdänsäure, Z. Anorg. Allgem. Chem., 1978, vol. 446, pp. 5–16.
Unger, B., Popp, P.B., Schade, U., and Hähnert, M., Reaction kinetics of SiO2 and ZnO-SiO2 sol-gel solutions, J. Non-Cryst. Solids, 1993, vol. 160, pp. 152–161.
Rakhimov, V.I., Rakhimova, O.V., and Semov, M.P., Kinetics of the early stages of the sol–gel process: II. Distribution of silica over molecular species, Glass Phys. Chem., 2008, vol. 34, no. 2, pp. 160–165.
Mavrodineanu, R., Shultz, J.I., and Menis, O., Accuracy in Spectrophotometry and Luminescence Measurements, Washington: National Bureau of Standards, 1972.
Flory, P.J., Principles of Polymer Chemistry, Ithaka: Cornell Univ. Press, 1953, ch. 9.
Iler, R.K., The Chemistry of Silica, New York: Wiley, 1979, part 1.
Funding
The work was supported by state assignment no. 0097–2015–0021 of the Program of Fundamental Research of the State Academies of Sciences (in 2013–2015 no. 01201353825, in 2016/2018 no. АААА-А16-116020210284-7, in 2019/2021 no. АААА-А19-119022290087-1) and supported in part by the Department of Chemistry and Materials Science, Russian Academy of Sciences (subject 2).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by E. Linnik
Rights and permissions
About this article
Cite this article
Rakhimova, O.V., Magomedova, O.S. & Tsyganova, T.A. Investigation of Hydrolytic Polycondensation in Systems Based on Tetraethoxysilane by DK-Spectrophotometry Method. Glass Phys Chem 45, 419–427 (2019). https://doi.org/10.1134/S1087659619060166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659619060166