Abstract
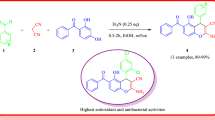
A simple, efficient, and green protocol has been developed for the synthesis of 2-amino-4-aryl-4H-chromene-3-carbonitriles via a one-pot three-component condensation of resorcinol, malononitrile, and substituted aromatic aldehydes in ethanol under reflux using InCl3 as a catalyst. This methodology has a number of advantages such as the use of a very small amount of catalyst, easy access, short reaction time, easy workup, high yields, and nontoxicity of the catalyst and solvent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The development of new methods for the synthesis of biologically active compounds is an important problem in current synthetic chemistry from both environmental and economic points of view. Chromene has emerged as a privileged scaffold for drug design and discovery and attracted great attention since many natural and synthetic chromene derivatives possess important biological properties such as antimicrobial [l], antiviral [2], antitumor [3], antivascular [4], and anticonvulsant activities [5]. They are also utilized in cosmetics, pigments, biodegradable agrochemicals, as well as photoactive materials [6]. Generally, the synthesis of 2-amino-4H-chromene involves cyclization of an aromatic aldehyde, malononitrile (or ethyl cyanoacetate), and enolizable CH-acidic compounds, e.g., phenol [7], resorcinol [8], 4-hydroxycoumarin [9], or dimedone [10].
A common method for the synthesis of chromenes is based on the condensation of aldehyde, phenol, and malononitrile in the presence of an organic base such as piperidine [11]. Several other reagents, such as Mg/Al hydrotalcite [12], KF or NaOAc [13], and basic ionic liquid [14] have been reported to effectively catalyze the one-pot synthesis of 2-aminochromenes. Most of these methods are characterized by long reaction times, harsh reaction conditions, low yields, and use of organic solvents.
RESULTS AND DISCUSSION
We initially focused on optimization of the reaction conditions. A mixture of resorcinol, malononitrile, and benzaldehyde in ethanol was refluxed in the presence of indium(III) chloride (15 mol %) as a model reaction (Scheme 1). Different polar and nonpolar solvent were tried, and ethanol was found to be the best solvent due to high reaction rate and high yield (Table 1, entry no. 6). The other polar protic solvents gave moderate yield (Table 1, entry no. 5), while the reaction in aprotic solvent like acetonitrile, DMF, THF, and methylene chloride was slower, which lead to lower yields (Table 1, entry nos. 1–4). The model reaction was also carried out using different amounts of the catalyst (Table 2). Excellent yield was achieved by using 15 mol % of InCl3 (Table 2, entry no. 6).
Under the optimized conditions (15 mol % InCl3, ethanol, reflux, 5 h), different substituted benzaldehydes 2a–2e were reacted with resorcinol (1) and malononitrile (3). Regardless of the substituent nature, either electron-withdrawing or electron-donating, the corresponding 2-amino-4H-chromene derivatives 4a–4e were obtained in good to excellent yields. The products were characterized by IR, 1H and 13C NMR, and mass spectra and elemental analyses.
The synthesized compounds were evaluated for their antimicrobial activity using the agar well diffusion method (Muller–Hinton agar medium). The results are collected in Table 3. Compounds 4a, 4c, and 4e showed good antibacterial activity against Staphylococcus aureus as compared to the standard drugs penicillin and streptomycin. Compounds 4a, 4b, and 4d showed good antibacterial activity against Escherichia coli, and compounds 4b, 4d, and 4e were active against Proteus vulgaris. Compounds 4c, 4d, and 4e showed good zones of inhibition against Aspergillus fumigatus, and 4b, 4d, and 4e showed good activity against Aspergillus niger. Nystatin was used as the standard antifungal drug.
All chemicals (analytical grade) were obtained from Sigma–Aldrich and Thomas Baker and were used without further purification. The reactions were carried out in dried glassware. The melting points were measured in open capillary tubes and are uncorrected. The IR spectra were recorded in KBr on a Perkin Elmer FTIR spectrometer. The mass spectra (electron impact) were run on a Shimadzu LCMS-2010EV instrument. The 1H and 13C NMR spectra were recorded on a Bruker Avance III HD spectrometer at 400 MHz for 1H using DMSO-d6 as a solvent and tetramethylsilane as an internal standard.
General procedure for the synthesis of 2-amino-4H-chromene derivatives 4a–4e. A mixture of resorcinol (10 mmol), malononitrile (10 mmol), aromatic aldehyde (10 mmol), and InCl3 (15 mol %) in ethanol was refluxed for 5 h. After completion of the reaction (TLC), the mixture was cooled to room temperature and poured into ice water, and the solid product was filtered off, washed with 50 mL of water, and recrystallized from ethanol. The spectral and analytical data for newly synthesized compounds 4b–4d are given below.
2-Amino-7-hydroxy-4-(4-methoxyphenyl)-4H-chromene-3-carbonitrile (4b). Yield 85%, mp 115–117°C. IR spectrum, ν, cm–1: 3420, 3350, 2192, 1660, 1580. 1H NMR spectrum, δ, ppm: 3.60 s (3H, OCH3), 4.66 s (1H, 4-H), 6.50–6.90 m (3H, Harom), 6.90 d (2H, Harom), 7.20 d (2H, Harom), 7.96 s (2H, NH2), 8.90 s (1H, OH). 13C NMR spectrum, δC, ppm: 161.4, 157.2, 152.5, 147.9, 143.1, 139.8, 135.5, 130.2, 126.0, 120.5, 114.3, 111.9, 102.5, 56.5, 51.2, 22.0. Mass spectrum: m/z 294 (Irel 100%) [M]+. Found, %: C 69.40; H 4.80; N 9.50. C17H14N2O3. Calculated, %: C 69.38; H 4.79; N 9.52.
2-Amino-4-(4-chlorophenyl)-7-hydroxy-4H-chromene-3-carbonitrile (4c). Yield 84%, mp 225–227°C. IR spectrum, ν, cm–1: 3450, 3340, 2196, 1650, 1566. 1H NMR spectrum, δ, ppm: 4.72 s (1H, 4-H), 6.40–6.95 m (3H, Harom), 7.20 d (2H, Harom), 7.50 d (2H, Harom), 7.90 s (2H, NH2), 8.92 s (1H, OH). 13C NMR spectrum, δC, ppm: 160.9, 156.8, 152.2, 148.0, 144.1, 139.0, 135.6, 130.2, 126.5, 120.5, 114.6, 112.0, 102.5, 56.2, 48.2. Mass spectrum: m/z 298 (Irel 100%) [M]+. Found, %: C 64.30; H 3.70; N 9.40. C16H11ClN2O2. Calculated, %: C 64.33; H 3.71; N 9.38.
2-Amino-4-(4-bromophenyl)-7-hydroxy-4H-chromene-3-carbonitrile (4d). Yield 88%, mp 230–232°C. IR spectrum, ν, cm–1: 3470, 3350, 2190, 1655, 1548. 1H NMR spectrum, δ, ppm: 4.70 s (1H, CH), 6.45–6.90 m (3H, Harom), 7.22 d (2H, Harom), 7.65 d (2H, Harom), 7.90 s (2H, NH2), 8.90 s (1H, OH). 13C NMR spectrum, δC, ppm: 160.5, 156.9, 152.0, 148.0, 144.5, 140.0, 135.4, 130.2, 126.5, 120.6, 114.0, 112.0, 102.0, 56.5, 48.2. Mass spectrum: m/z 342 (Irel 100%) [M]+. Found, %: C 56.00; H 3.25; N 8.15. C16H11BrN2O2. Calculated, %: C 56.00; H 3.23; N 8.16.
CONCLUSIONS
An operationally simple, inexpensive, efficient, and eco-friendly synthesis of 2-amino-4H-chromene derivatives by one-pot three-component condensation has been developed. The procedure offers several advantages, including improved yields, cleaner reactions, and low cost, which makes it a useful and attractive strategy in view of economic and environmental aspects. Some of the synthesized compounds showed a good activity against gram-positive and gram-negative bacterial strains.
REFERENCES
Dongamanti, A., Bommidi, V.L., Sidda, R., Arram, G., and Shaik, A., Chem. Heterocycl. Compd., 2015, vol. 51, p. 462. https://doi.org/10.1007/s10593-015-1719-0
Cinzia, C., Luca, P.M., and Nicoletta, D., Bioorg. Med. Chem., 2014, vol. 22, p. 1201. https://doi.org/10.1016/j.bmc.2013.11.054
Tilak Raj, R.K., Bhatia, A.M., Sharma, A.K., and Saxena, M.P., Eur. J. Med. Chem., 2010, vol. 45, p. 790. https://doi.org/10.1016/j.ejmech.2009.11.001
Gourdeau, H., Leblond, L., Hamelin, B., Desputeau, C., Dong, K., Kianicka, I., Custeau, D., Boudreau, C., Geerts, L., Cai, S.-X., Drewe, J., Labrecque, D., Kasibhatla, S., and Tseng, B., Mol Cancer Ther., 2004, vol. 3 p. 1375. https://doi.org/10.1158/1535-7163.1375.3.11
Mashooq, A.B., Nadeem, S., and Suroor, A.K., Acta Pol. Pharm., 2008, vol. 65, p. 235. PMID 18666431
Ebtisam, A.A.H., Mohamed, H.E., Amel, G.A.E., and Fathy Mohamed A.A.T. Heterocycles, 1987, vol. 26, p. 903. https://doi.org/10.3987/R-1987-04-0903
Magar, R.L., Thorat, P.B., Jadhav, V.B., Tekale, S.U., Dake, S.A., Patil, B.R., and Pawar, R.P., J. Mol. Catal. A: Chem., 2013, vol. 374, p. 118. https://doi.org/10.1016/j.molcata.2013.03.022
Javad, S., Marzieh, H., and Zohre, Z., Arab. J. Chem., 2017, vol. 10, p. 2994. https://doi.org/10.1016/j.arabjc.2013.11.038
Sedaghat, M.E., Rajabpour, M.B., Nazarifar, M.R., and Farhadi, F., Appl. Clay Sci., 2014, vol. 95, p. 55. https://doi.org/10.1016/j.clay.2014.02.016
Bagher, A., Mohammad, S., and Mehdi, A., Res. Chem. Intermed., 2016, vol. 42, p. 3413. https://doi.org/10.1007/s11164-015-2220-1
Elagamey, A.G.A., El-Taweel, F.M.A., Khodeir, M.N.M., and Elnagdi, M.H., Bull. Chem. Soc. Jpn., 1993, vol. 66, p. 464. https://doi.org/10.1246/bcsj.66.464
Mandar, P.S., Siddheshwar, K., and Shriniwas, D.S., Tetrahedron Lett., 2009, p. 719. https://doi.org/10.1016/j.tetlet.2008.11.114
Elinson, M.N., Ilovaisky, A.I., Merkulova, V.M., Belyakov, P.A., Chizhov, A.O., and Nikishin, G.I., Tetrahedron, 2010, vol. 66, p. 4043. https://doi.org/10.1016/j.tet.2010.04.024
Kai, G., Hua-Lan, W., and Dong-Fang, Z.L., Catal. Commun., 2008, vol. 9, p. 650. https://doi.org/10.1016/j.catcom.2007.07.010
ACKNOWLEDGMENTS
The authors are grateful to Principal, Yeshwant Mahavidyalaya, Nanded, for providing laboratory facilities, UGC, New Delhi (File no.41-230/2012) (SR) for financial support, and The Director, Punjab University, Chandigarh for providing spectra.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Choudhare, S.S., Bhosale, V.N. & Chopade, M. Synthesis of 2-Amino-4H-chromene Derivatives Using InCl3 and Their Antimicrobial Evaluation. Russ J Org Chem 58, 913–916 (2022). https://doi.org/10.1134/S1070428022060227
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022060227