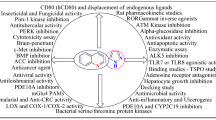
Abstract
Pyrrolones and pyrrolidinones are five-membered heterocycles and are versatile lead compounds for designing powerful bioactive agents. Pyrrolone and pyrrolidinone moieties are among the most essential heterocyclic pharmacophores inducing prominent pharmaceutical effects. Therefore, researchers paid attention to synthesize various pyrrolone and pyrrolidinone derivatives. Numerous methods for the synthesis of pyrrolones and pyrrolidinones offer a great scope in the field of medicinal chemistry. This attractive group of compounds has diverse biological activities like antimicrobial, anti-inflammatory, anticancer, antidepressant, anticonvulsant, etc. The purpose of this review is to classify broad information on the chemistry and pharmaceutical effects of pyrrolones and pyrrolidinones to open a new perspective for future studies. It is clear that a wide spectrum of pyrrolones and pyrrolidinone derivatives have been synthesized and that most of them have various significant biological activities. Thus, these derivatives can be used for the future development of novel compounds active against different infections and diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Introduction
3.2. Anticancer Activity
3.5. Anti-HCV Activity
5. Conclusions
1. INTRODUCTION
Rising concern has been paid to the synthesis of N-heterocyclic compounds due to their useful biological activities and their role as intermediates in medicinal and organic chemistry [1]. Heterocyclic compounds are the most essential in the discovery of novel drugs. Various compounds such as alkaloids, amino acids, hemoglobin, vitamins, hormones, most synthetic drugs, and dyes contain heterocyclic ring systems. Nitrogen-containing heterocyclic compounds have been the topic of ample research due to their diverse and prominent biological, agrochemical, and synthetic applications. Pyrrolidin-2-one is a five-membered lactam present in both natural and synthetic compounds [2]. The presence of a pyrrol-2-one fragment in drugs and natural compounds has gained significant attention in the development of novel methods of their synthesis [3]. Pyrrolidin-2-ones have been used in the synthesis of various alkaloids [4] and unusual β-amino acids such as statin and its derivatives [5].
1,3-Dihydro-2H-pyrrol-2-one (pyrrolone) derivatives exhibit diverse biological activities such as anti-inflammatory, analgesic [6], antibacterial [7] cardiotonic [8], antimycobacterial [9], antidepressant [10], antiviral [11], antitumor [12], and other activities [13–18]. Pyrrolones can be synthesized by nucleophilic substitution from the corresponding furanones [6], as well as by different methods based on heterocyclization of various Schiff bases with maleic anhydride [19, 20] and the reaction of furanone derivatives with amines [21–23].

2. PYRROLONE AND PYRROLIDINONE DERIVATIVES AND THEIR BIOLOGICAL USES
Compounds containing a pyrrolone moiety have wide clinical applications. For instance, althiomycin is a naturally occurring alkaloid produced by Streptomyces althioticus and is used as an antibiotic which inhibits protein synthesis [7]. The pyrrolinone system is also present in natural compounds such as vitamin B12, prodigiosin, bile pigments, and some antibiotics like distamycin [24]. Various pyrrolone and pyrrolidinone derivatives are structural fragments of plentiful natural compounds such as holomycin, thiolutin [25], bilirubin [26], ypaoamide [27], lactacystin [28], indolocarbazole alkaloids (staurosporine) [29], oteromycin [30], pyrrocidines A and B [31], thiomarinol A4 [32], quinolactacin C [33], salinosporamide A [34], (–)-azaspirene (angiogenesis inhibitor isolated from the fungus Neosartorya sp.) [35]. Oteromycin is the ETB receptor antagonist (IC50 = 2.5 pM) [30] (Scheme 1).
2-Oxodihydropyrrole ring is present in some alkaloids possessing diverse pharmaceutical activities. Pyrrolone derivatives are used as optoelectronic materials [36], PI-091 is a platelet aggregation inhibitor [37], and thiomarinol A4 is a potent antibiotic. Inhibitors of HIV integrase [38], cardiac cyclic AMP phosphodiesterase (PDE) [39], and vascular endothelial growth factor receptor [40], neuritogenic [41], pesticidal [42], antibacterial, antifungal, and nootropic agents [43], peptidomimetics [44], synthetic intermediates [45], DNA polymerase inhibitors [46], caspase-3 inhibitors [47], anticancer and cytotoxic agents [48], human cytomegalovirus (HCMV) protease inhibitors [49], human cytosolic carbonic anhydrase isozyme inhibitors [50], antibiotics [51], and annexin A2–S100A10 protein interaction inhibitors [52] were found among pyrrolone derivatives. Cotinine is an alkaloid present in tobacco and the main metabolite of nicotine [53]. Ethosuximide is a succinimide anticonvulsant used mainly in the treatment of the absence of seizures [54].
Despite their diverse usages, existing ways for their synthesis are limited mainly to multicomponent reactions (MCRs) [55]. N-Alkyl-5-methylpyrrolidin-2-ones can be synthesized by reductive amination of levulinic acid or its esters. They are widely used as surfactants, solvents, intermediates for pharmaceuticals and agrochemicals [56]. N-Vinylpyrrolidone (NVP) has been studied for its usages in various fields [57] due to its good biocompatibility, hydrophilic character, and low cytotoxicity [58].
Pyrrolidinone cognition enhancers like piracetam and oxiracetam (Scheme 2) constitute a separate group due to their unique selectivity for brain areas involved in the procedures of acquiring knowledge and memory processes and safe profile [59]. These features make them suitable for chronic therapy in aged patients. Poly(vinylpyrrolidone) (PVP) is an important water-soluble polymer having diverse applications ranging from pharmacology (as a binder in tablets) to nanoparticles and membranes for water purification [60]. Blending with PVP improves the anti-fouling properties of biomedical membrane materials used in plasma separation and hemodialysis [61]. The R enantiomer of the marine natural product (S)-ypaoamide (Scheme 1) was obtained by asymmetric synthesis. The 3-pyrrolin-2-one moiety is the most important fragment responsible for the immunosuppressive effect [62]. One-pot multicomponent synthesis of substituted 3-pyrrolin-2-ones 1 using citric acid as a green catalyst in a green solvent under ultrasound irradiation has been reported [63].
Optically pure forms (98%) of 1-(2-tert-butylphenyl)-5-(methoxymethyl)pyrrolidin-2-one (2) and 1-{2-[tert-butyl(diphenyl)siloxy]phenyl}-5-(methoxymethyl)pyrrolidin-2-one (3) (including atropisomerism) were prepared from (S)-5-(methoxymethyl)butyrolactone in a stereoselective fashion [64]. Monocarbonyl analogs of cyclic imides, N-phenylpyrrolidin-2-ones and N-phenyldihydro-1H-pyrrol-2-ones 4–6, acted as potential protoporphyrinogen oxidase (PPO) inhibitors. The in vitro assay showed that most of the synthesized compounds have good to excellent PPO inhibition activity and significant herbicidal activity [65] (Scheme 2).
Homochiral 5-methylidene-1,5-dihydro-2H-pyrrol-2-one derivatives 7 and 8 were synthesized from amino acid esters, amino alcohols, and chiral amines via photooxygenation [51] (Scheme 3). These compounds are useful precursors to various bioactive compounds like alkaloids and unusual amino acids. The multifunctional character of these derivatives can contribute to multiple stereoselective reactions like conjugate additions, allylic substitutions, and cycloadditions [66].
Kawasuji et al. [38] reported the synthesis of a series of HIV inhibitors 9 containing a 3-hydroxy-1,5-dihydro-pyrrol-2-one moiety as a developmental derivative of 2-hydroxy-3-heteroaryl acrylic acid inhibitors (HHAAs). The cyclic modification of the chelating entity of HHAA provided a favorable configuration for the coordination of two metal ions in HIV, which improved not only enzymatic evaluation but also antiviral cell-based assays in many cases [38]. Peptidomimetic analogs 10 were prepared from amino acid-derived tetramic acids as the key starting materials [44] (Scheme 4).
Tetra- and pentasubstituted polyfunctional dihydropyrrole derivatives 14 and 15 were synthesized by the reaction of but-2-ynedioates, aldehydes, and amines at room temperature or 70°C. Their in vitro pharmacological evaluation against HIV-1 showed a considerable effect with IC50 values in the micromolar range (38– 58 µM) [67]. Dihydropyrrole derivatives 16 and 17 exhibited inhibitory activity against caspase-3 with IC50 values ranging from 5 to 20 µM; one compound (IC50 = 5.27 µM) can be regarded as a superior caspase-3 inhibitor [47]. The synthesis of polyfunctional 2-oxodihydropyrrole derivatives 18 by one-pot four-component domino reaction of dialkyl acetylenedicarboxylate, amine, and formaldehyde at room temperature was reported [68]. N-Substituted 5-methylpyrrolidin-2-one derivatives 19 were synthesized by one-pot reaction of ethyl levulinate and nitro compounds in presence of nanosized platinum catalyst [69] (Scheme 5).
1H-Pyrrol-2(5H)-ones 20 were synthesized from α,β-unsaturated ketones and ethyl nitroacetate under Paal–Knorr conditions [70] (Scheme 6).
N-Acyl-5-oxopyrrolidine-2-carbonitriles 21 were prepared by reacting 5-oxopyrrolidine-5-carboxamide (pyroglutamide) with trimethylchlorosilane in presence of zinc chloride as a catalyst [71]. Compounds 22 and 23 were claimed as potentially useful in the treatment of a wide range of diseases like schizophrenia, Alzheimer’s disease, Parkinson’s disease, anxiety, depression, stroke, down syndrome, traumatic brain injury, and normal aging. The neurogenic features of the compounds were tested on the proliferation of human embryonic stem cells. The compound efficacy was measured by the increase in cells based on ATP levels. Among the compounds tested, 22 showed the strongest neurogenic features [72]. Six derivatives of 3,3-diphenylpyrrolid-2-one were synthesized and tested for anticonvulsant activity. Among them, 2-imino-1,5-dimethyl-3,3-diphenylpyrrolidine hydrochloride 24 was effective in mice against maximal electroshock-induced seizures [73]. The anticonvulsant effects of these derivatives depended on the substituent at the 3-position, and the presence of an asymmetric center generally increased the anticonvulsant activity [74]. N-Aryl-3-amino-2-oxo-1,5-dihydropyrrole-4-carboxylates 25 were synthesized by vitamin B12-catalyzed condensation between formaldehyde, dialkyl acetylenedicarboxylates, and amines at ambient temperature in ethanol [75]. Pyrrolidinone derivatives 26 were regioselectively synthesized by a one-pot intermolecular reductive coupling of acrylamides and nitriles in the presence of a cobalt catalyst [76] (Scheme 7).
The synthesis and hybridization activities of four stereoisomeric adenine pyrrolidinone PNA analogs toward DNA, RNA, and PNA (peptide nucleic acid) were reported [77] (Scheme 8). A special asymmetric synthesis of pyrrolidinone derivatives 27 starting from 3-acylpropionic acids and N-substituted pyrrolidinone was accomplished by reduction of chiral bicyclic lactams 28 which were prepared from (R)-phenylglycinol using triethylsilane and titanium tetrachloride [78]. A series of dihydro-1H-pyrrolo[1,2-a]imidazole-2,5(3H,6H)-diones 29 were prepared and were found to exhibit anti-amnesic activity. The unsubstituted compound (29, R1 = R2 = R3 = H, n = 1, dimiracetam) is 10–30 times stronger than oxiracetam [59] (Scheme 9). N-Arenesulfonyl-5-oxopyrrole-2,3-dicarboxylates 30 were synthesized by the reaction of dialkyl acetylenedicarboxylates, ethyl chlorooxoacetate, and arenesulfonamides in the presence of Et3N and Ph3P under mild conditions [79] (Scheme 9).
The synthesis of thiolutin (Scheme 1) and its derivatives was achieved by reacting methoxycarbonylacetyl chloride and N-methyl-1-ethoxycarbonyl-2-diethoxyethylamine [80]. Holomycin (Scheme 1) and its derivatives were synthesized using S-benzyl-L-cysteine ethyl ester as a starting material [81]. Epolactaene (31), a neurogenic substance in human neuroblastoma cells, and its derivatives 32–34 (Scheme 10) showed DNA polymerase activities on mammalian and human DNA topoisomerase II with IC50 values of 25, 94, and 10 µM, respectively [46].
A series of substituted 6-amino-4H-[1,2]dithiolo[4,3-b]pyrrol-5-ones 35 exhibited cytotoxicity effect [48]. An effective synthetic route to functionalized dihydropyrrol-2-one derivatives 36 via domino reaction of ethyl glyoxylate with acetylenedicarboxylate and 2 equiv of aromatic amines in the presence of benzoic acid was described [82] (Scheme 11).
2-Oxo-2,5-dihydro-1H-pyrrole-4-carboxylic acid alkyl esters 37 were prepared by a novel, facile, and general approach involving multicomponent reaction of aldehyde, amine, and dialkyl acetylenedicarboxylate in the presence of reusable TiO2 nanopowder [83]. Lactic acid was used as a more valuable and greener additive for the one-pot synthesis of pyrrole derivatives 38 in ethanol at ambient temperature [84] (Scheme 12).
Donohoe et al. [85] reported the asymmetric synthesis of the fully elaborated pyrrolidinone core of the β-lactone/γ-lactam antibiotic oxazolomycin A. The procedure included the Birch reduction of an aromatic pyrrole nucleus, RuO4-catalyzed pyrrolidine oxidation, and diastereoselective organocerium addition to an aldehyde (Scheme 13).
3. BIOLOGICAL PROPERTIES
Various pyrrol-2-one derivatives exhibited diverse types of pharmacological activities against different types of diseases or disorders.
3.1. Antimicrobial Activity
Pyrrole-2-one derivative 39 exhibited significant activity against S. aureus with a minimum inhibitory concentration (MIC) of 6.5 μg/mL and good activity against E. coli (MIC 15 μg/mL) [86]. 2(3H)-Pyrrolone derivatives were evaluated as antibacterial agents. Compound 40 showed significant antibacterial activity against S. aureus, E. coli, and P. aeruginosa at a level comparable to ciprofloxacin (MIC 6.25 µg/mL) [87]. A series of indolylpyrrolones displayed antibacterial activity. For instance, compound 41 was equipotent to chloramphenicol against E. coli with a MIC value of 2.5 µg/mL [88]. A series of pyrrolone and N-benzylpyrrolone derivatives were evaluated for antibacterial activity, and compound 42 exhibited the strongest antibacterial effect against E.coli and P. aeruginosa (MIC 6.25 µg/mL) and S. aureus (MIC 12.5 µg/mL) compared to ciprofloxacin (MIC 6.5 µg/mL) [89]. Among the quinolinyl pyrrolone series, compound 43 showed the highest antibacterial activity against E. coli and P. aeruginosa (MIC 12.5 µg/mL) and S. aureus (6.25 µg/mL) [90]. N-Benzylpyrrolone derivative 44 displayed remarkable antibacterial activity against S. aureus and E. coli with inhibition zone diameters of 5 and 7 mm, respectively, compared to penicillin (32 and 15 mm, respectively) [91]. 5-Phenoxyphenylpyrrol-2-one derivatives 45 and 46 showed antimicrobial activity against S. aureus (MIC 20 and 15 µg/mL, respectively) and C. albicans (MIC 10 µg/mL) [92]. Compound 47 showed the most promising antimycobacterial activity with IC50 of 11.34 µg/mL [93] (Scheme 14).
Pyrrolidin-2-ones possess both antibacterial and antifungal activity. Aqueous Lutrol® F127 system comprising N-methylpyrrolidin-2-one (NMP) showed antimicrobial effect against S. aureus, E. coli, and C. albicans in a dose-dependent manner with respect to NMP [94]. 2-[1,3-Diphenyl-1H-pyrazol-4-yl]-5-oxopyrrolidine-3-carboxylic acids 48 containing a benzenesulfonamide moiety exhibited similar or better antibacterial activity than those of ampicillin, tetracycline, gentamycin, and chloramphenicol against B. subtillis, S. aureus, E. coli, and P. aeruginosa [95]. N-Vinylpyrrolidone (NVP)–acrylic acid (AA) co-polymer 49 was synthesized and grafted with N,N-diethylaminoethanol through the carboxylic acid group to form an ester. The resulting copolymer showed antibacterial activity against gram-negative Klebsiella aerogenes NCIM-2098, Pseudomonas desmolyticum NCIM-2028, and E. coli NCIM-5051, as well as gram-positive S. aureus NCIM-5022. A significant antibacterial effect was observed at a copolymer dose of 150 µg in all bacterial pathogens tested [96] (Scheme 15).
A series of amphiphilic poly(N-vinylpyrrolidone)(PNVP)-b-poly(D,L-lactide)-b-PNVP triblock copolymers 50 have been synthesized, and doxorubicin (DOX) was loaded into the block copolymer micelles with a loading efficiency of 37.5%. The antibacterial properties of DOX-loaded micelles were found to be significantly more effective with respect to free DOX [97]. Poly(vinyl alcohol)/poly(vinyl pyrrolidone) (PVA/PVP) hydrogel was obtained by using γ-irradiation method. The antimicrobial effect of PVA/PVP hydrogels was tested against S. aureus, P. aeruginosa, B. subtilis, and E. coli, as well as against the fungi Aspergillus fumigatus, Penicillium italicum, Syncephalastrum racemosum, and C. albicans [98]. 3,3-Difluoropyrrolidin-2-one derivatives 51 were synthesized by the reaction of ethyl 2,2-difluoro-4-iodo-4-(trimethylsilyl)butanoate with amines and were found to be biologically significant [99] (Scheme 16).
Pyrrocidines A and B, the two antibiotics including 13-membered macrocycles, were isolated from the fermentation broth of a fungus, LL-Cyan426; pyrrocidine A showed a stronger effect against gram-positive bacteria than that of pyrrocidine B. It was also effective against C. albicans [31]. The pyrrolidinone moiety was found in other antifungal agents such as talaroconvolutin A and the novel platelet-activating factor acetyltransferase inhibitor ZG-1494 (Scheme 17), but the 13-membered macrocycle including phenyl, ether, pyrrolidinone, and ketone moieties as in talaroconvolutin A is the prime instance discovered in natural products [100, 101].
A series of GEQ (Genz-10850) analogs were synthesized in a few steps to afford pyrrolidinone and pyrrolidine derivatives 52. These compounds were tested against InhA, an essential target for Mycobacterium tuberculosis (M.tb) survival. Compounds 52 were found to be quite active with MIC values of 1.4 and 2.8 µM respectively [102]. Photo-cross-linked quaternized chitosan analogs 53 (Scheme 18) prepared by electrospinning exhibited a strong antibacterial effect against gram-positive S. aureus and gram-negative E. coli. These materials can be used for wound dressing. Poly(vinyl pyrrolidone) (PVP) has numerous applications in the biomedical field due to its useful features such as non-toxicity, high hydrophilicity, biocompatibility, excellent complexation features, and film-forming ability [103].
3.2. Anticancer Activity
3-Hydroxy-1-(4-methylphenyl)-5-(4-nitrophenyl)-4-pivaloyl-2,5-dihydro-1H-pyrrol-2-one (54) exhibited antitumor activity against lung cancer with growth inhibition of 55% and CNS cancer with growth inhibition of 67% [104]. A series of 5-aryl-5-hydroxypyrrol-2-ones like 55 inhibited the growth of colon and pancreatic cancer models and acted as cholecystokinin-1 receptor antagonists with IC50 of 0.008 and 0.4 μM against CCK-A and CCK-B, respectively; this compound was more effective than lorglumide (IC50 0.17 and >10 µM, respectively) [105]. A series of pyrrolone derivatives showed anticancer activity against HePG2, HCT116, and PC3 cancer cell lines. Compound 56 exhibited comparable activity to doxorubicin against Hep-G2 cancer cell line. Compounds 57 and 58 showed good cytotoxic activity against Hep-G2 cancer cell line with IC50 of 11.47 and 7.11 μM, respectively, compared to paclitaxel (IC50 0.73 μM). These two compounds displayed promising inhibition for tubulin polymerase [106]. A series of indolyl-pyrrolone derivatives were tested as PIM1 kinase inhibitors, and compound 59 showed the highest activity with ID50 of 4.5 μM [107] (Scheme 19).
A series of helicid–pyrrolidone derivatives 60 (Scheme 20) were tested for their anticancer effect against human SKOV3 cells. Two derivatives showed a high anticancer effect against this cell line with an IC50 range of 0.22 to 6.5 μM, respectively, and were more potent than 17AAG, the heat shock protein 90 (Hsp90) inhibitor [62]. 1-Substituted 4-aroyl-3-hydroxy-5-phenyl-1H-pyrrol-2(5H)-one derivatives 61 (Scheme 20) were synthesized as Annexin A2-S100A10 protein inhibitors. Selected analogs interrupted the complex structure of Annexin A2 and S100A10 both in broken cell preparation and inside MDA-MB-231 breast cancer cells [52]. The toxic effect of NEP (N-ethylpyrrolidone) was exhibited when used by gavage to Sprague-Dawley rats at doses of 5, 50, and 250 mg/kg/day for four weeks. Finally, 28 days of repeated oral exposure to NEP at doses of up to 250 mg/kg/day resulted in mild renal and hepatic effects in rats. The adverse effects consisted of a reversible reduction in body heaviness gain and a growth in urine volume [108]. Dithiolopyrrolone derivatives 62 constitute a class of strong natural antibiotics effective against both gram-negative and gram-positive bacteria. They include a 4H-[1,2]dithiolo[4,3-b]pyrrol-5-one chromophore, and have recently attracted growing attention in synthetic and biological studies as antiproliferative factors [109]. Compound 63 was selected for its anticancer effect tested on human SKMEL-28 V+ xenografts both in monotherapy and in combination with Taxol or Cisplatin, where potent effectiveness was found [48] (Scheme 20).
More than 300 nitrogen-containing natural products have been isolated and identified from marine cyanobacteria [110]. Among these, microcolins A and B isolated from a Venezuelan sample of the blue-green algae Lyngbya majuscule [111] showed very effective immunosuppressive and antiproliferative effects [112]. Jamaicamides A–C belong to a class of strong neurotoxins isolated from the Jamaican strain of L. majuscule [48, 113] (Scheme 21).
3.3. Anti-inflammatory Activity
A series of pyrrol-2-one exhibited anti-inflammatory activity, and compound 64 has comparable activity to diclofenac [86]. Quinolinylpyrrolone derivatives were tested as anti-inflammatory agents using carrageenan-induced paw edema. Compounds 65 and 66 produced 53% and 63% inhibition, respectively. The results indicated that the conversion of secondary NH group into tertiary moiety by introducing a benzyl substituent increased the anti-inflammatory activity [90]. Compounds 67 and 68 showed an edema inhibition percentage comparable to that of diclofenac (71.47, 76.22, and 80.98%), respectively. These compounds suppressed the TNF-a level by 60.90 and 65.03%, respectively, compared to indomethacin (68.40%) [114]. A series of furan-2(3H)-ones and their 1-benzylpyrrol-2(3H)-one analogs, in particular compounds 69 and 70 displayed comparable anti-inflammatory activity to ibuprofen with inhibition % of 88.88, 89.50, and 89.50, respectively. These two compounds also showed superior gastric safety with protection % of 57.83 and 59.03 compared to ibuprofen (65.06%) and reduced lipid peroxidation to a greater extent than did the reference drug [9, 115]. Pyrrolone derivatives 71 and 72 exhibited significant anti-inflammatory activity which was comparable to that of diclofenac and a twice as high safety profile as that of diclofenac (1.3, 1.3, and 2.6, respectively) [116] (Scheme 22).
3.4. Antidepressant Activity
Pyrrolone 73 exhibited significant antidepressant potency, selectivity, and pharmacokinetic profile and showed nanomolar affinity to 5-HT2C receptor [117] (Scheme 23).
3.5. Anti-HCV Activity
A series of pyrrolone derivatives were tested for their antiviral activity against the hepatitis C virus (HCV). Compound 74 showed moderate interference with the helicase unwinding activity with IC50 of 438 μM and proved to be less potent than primuline (IC50 10 µM) [11]. A series of bicyclic octahydrocyclohepta[b]pyrrol-4(1H) one derivatives exhibited antiviral activity against HCV. For example, N-tosyl derivative 75 was characterized by EC50 values of 1.8 and 4.5 µM in genotypes 1b and 2a, respectively. This compound did not affect HCV NS5B, IRES, and NS3 helicase [118] (Scheme 24).
4. INDUSTRIAL APPLICATIONS
The main features of 1-vinylpyrrolidin-2-one (76) are its polarity characteristics and polymerizability to form soluble poly(vinylpyrrolidone) (PVP) or insoluble polyvinylpolypyrrolidone (PVPP, a highly cross-linked modification of PVP). PVP-based products are used in various fields are produced on large scale. PVP is used as an excipient in pharmacological formulations like tablets, food additive, and UV-durable inks. 1-Vinylpyrrolidin-2-one is capable of permeating skin [57, 58]. An analytical procedure has been developed for the determination of 1-vinyl-2-pyrrolidone–mercapturic acid (VPMA) in urine using electrospray liquid chromatography–tandem mass spectrometry (ESI-LC/MS) column switching approach [119].

N-Ethyl-2-pyrrolidone (NEP, 77) is an industrial chemical used as a solvent, catalyst, and surfactant. It was proposed as a substitute for its analog, N-methyl-2-pyrrolidone (NMP, 78), in many areas of usage, including coatings industry and cleaning of metals, glass, and plastics [108]. N-Methyl-2-pyrrolidone (NMP) is the 4-methylaminobutyric acid lactam. It is a colorless thermally stable liquid with low viscosity, low toxicity, and good biocompatibility. The dissolving power of NMP is similar to those of ethanol and dimethyl sulfoxide (DMSO). NMP increases transdermal sorption of some drugs like ibuprofen, flurbiprofen, phenolsulfonphthalein, and estradiol. It is isolated from a marine sponge for biosynthesis [120]. NMP can be used in the parenteral preparation of drugs since its increased solubilizing potency and low viscosity are the main factors for fine-gauge needles or microcatheters [121]. It is an important solvent used in extraction, purification, and crystallization of drugs [122]. NMP is used in nanoemulsions for the transdermal delivery of granisetron hydrochloride [123]. Many drugs contain NMP in their topical formulations as an absorption improver, in particular fluoxetine hydrochloride [124], lidocaine, granisetron hydrochloride [122], griseofulvin, [125] insulin [126], estradiol [125–127], bupranolol [128], spantide II [129], levonorgestrel [127], luteinizing hormone-releasing hormone [130], ibuprofen, flurbiprofen [131], and morphine hydrochloride. An additional syringe wash with NMP can improve the stability of injections using gas chromatography syringes by getting better peak symmetry and reproducibility [132].
5. CONCLUSIONS
Pyrrolidinone derivatives are regularly investigated, and this review mainly focused on different significant biological activities of pyrrolidinone derivatives such as anti-inflammatory, antibacterial, antimicrobial, anticoagulant, antihypertensive, antimycobacterial, and most importantly their anticancer activities [133, 134]. Medicinal chemists from all over the world have synthesized different substituted pyrrolone derivatives to explore their biological activity and their drug target. Despite the known biological activities of these scaffolds, there is a direction in the future toward the use of this nucleus in the design of novel molecules with effective biological activities.
REFERENCES
Zhang, P.Z., Zhou, S.F., Li, T.R., and Jiang, L., Chin. Chem. Lett., 2012, vol. 23, p. 1381. https://doi.org/10.1016/j.cclet.2012.10.024
Priebbenow, D.L. and Bolm, C., RSC Adv., 2013, vol. 3, p. 10318. https://doi.org/10.1039/C3RA41527A
Bai, W.-J., Jackson, S.K., and Pettus, T.R., Org. Lett., 2012, vol. 14, p. 3862. https://doi.org/10.1021/ol301556a
Casiraghi, G., Spanu, P., Rassu, G., Pinna, L., and Ulgheri, F., J. Org. Chem., 1994, vol. 59, p. 2906. https://doi.org/10.1002/1521-3773
Jouin, P., Castro, B., and Nisato, D., J. Chem. Soc., 1987, p. 1177. https://doi.org/10.1039/P19870001177
Li Petri, G., Raimondi, M.V., Spanò, V., Holl, R., Barraja, P., and Montalbano, A., Top. Curr. Chem., 2021, vol. 379, article no. 34. https://doi.org/10.1007/s41061-021-00347-5
Zarantonello, P., Leslie, C.P., Ferritto, R., and Kazmierski, W.M., Bioorg. Med. Chem. Lett., 2002, vol. 12, p. 561. https://doi.org/10.1016/s0960-894x(01)00802-2
Abdelbaset, M.S., Abdel-Aziz, M., AbuoRahma, G.E.A., Ramadan, M., and Abdelrahman, M.H., J. Adv. Biomed. Pharm. Sci., 2019, vol. 2, p. 19.
Khokra, S.L., Khan, S.A., Choudhary, D., Hasan, S.M., Ahmad, A., and Husain, A., Anti-Inflammatory Anti-Allergy Agents Med. Chem., 2016, vol. 15, p. 54. https://doi.org/10.2174/1871523015666160618113204
Lattmann, E.L., Sattayasai, J., Narayanan, R., Ngoc, N., Burrell, D., Balaram, P.N., Palizdar, T., and Lattmann, P., MedChemComm 2017, vol. 8, p. 680. https://doi.org/10.1039/C6MD00707D
Bassetto, M., Leyssen, P., Neyts, J., Yerukhimovich, M.M., Frick, D.N., and Brancale, A., Bioorg. Med. Chem. Lett., 2017, vol. 27, p. 936. https://doi.org/10.1016/j.bmcl.2016.12.087
Ali, Y., Alam, M.S., Hamid, H., and Hussain, A., Orient. J. Chem., 2014, vol. 30, p. 1. https://doi.org/10.13005/ojc/300101
Tanwar, G., Mazumder, A.G., Bhardwaj, V., Kumari, S., Bharti, R., Yamini, Y., Singh, D., Das, P., and Purohit, R., Sci. Rep., 2019, vol. 9, p. 7904. https://doi.org/10.1038/s41598-019-44264-6
Zacarías, N.V.O., van Veldhoven, J.P.D., Portner, L., van Spronsen, E., Ullo, S., Veenhuizen, M., van der Velden, W.J.C., Zweemer, A.J.M., Kreekel, R.M., Oenema, K., Lenselink, E.B., Heitman, L.H., and Ijzerman, A.P., J. Med. Chem., 2018, vol. 61, p. 9146. https://doi.org/10.1021/acs.jmedchem.8b00605
Sweeney, N.L., Hanson, A.M., Mukherjee, S., Ndjomou, J., Geiss, B.J., Steel, J., Frankowski, K.J., Li, K., Schoenen, F.J., and Frick, D.N., Infect. Dis., 2015, vol. 1, no. 3, p. 140. https://doi.org/10.1021/id5000458
Murugesan, D., Kaiser, M., White, K.L., Norval, S., Riley, J., Wyatt, P.G., Charman, S.A., Read, K.D., Yeates, C., and Gilbert, I.H., ChemMedChem, 2013, vol. 8, no. 9, p. 1537. https://doi.org/10.1002/cmdc.201300177
Murugesan, D., Mital, A., Kaiser, M., Shackleford, D.M., Morizzi, J., Katneni, K., Campbell, M., Hudson, A., Charman, S.A., Yeates, C., and Gilbert, I.H., J. Med. Chem., 2013, vol. 56, p. 2975. https://doi.org/10.1021/jm400009c
Smith, C.R., Bunnelle, E.M., Rhodes, A.J., and Sarpong, R., Org. Lett., 2007, vol. 9, p. 1169. https://doi.org/10.1021/ol0701971
Kumar, D., Singh, A., and Walia, Y.K., Asian J. Adv. Basic Sci., 2014, vol. 2, p. 40.
Parmar, K., Sutariya, S., Shukla, M., and Goswami, K., J. Chem. Pharm. Res., 2012, vol. 4, p. 3478.
Khan, M., Husain, A., and Sharma, S., Indian J. Chem., Sect. B, 2002, vol. 41, p. 2160.
Afzal, O., Altamimi, A.S.A., Shahroz, M.M., Sharma, H.M., Riadi, Y., and Hassan, M.Q., Molecules, 2021, vol. 26, article no. 2389. https://doi.org/10.3390/molecules26082389
Kennedy, A.D., Pappan, K.L., Donti, T., Delgado, M.R., Shinawi, M., Pearson, T.S., Lalani, S.R., Craigen, W.J., Sutton, V.R., Evans, A.M., Sun, Q., Emrick, L.T.E., and Elsea, S.H., Front. Neurosci., 2019; vol. 13, article no. 394. https://doi.org/10.3389/fnins.2019.00394
Ayikpoe, R., Salazar, J., Majestic, B., and Latham, J.A., Biochemistry, 2018, vol. 57, p. 5379. https://doi.org/10.1021/acs.biochem.8b00816
Ettlinger, L., Gäumann, E., Hütter, R., KellerSchierlein, W., Kradolfer, F., Neipp, L., Prelog, V., and Zähner, H., Helv. Chim. Acta, 1959, vol. 42, p. 563. https://doi.org/10.1002/hlca.19590420225
Chen, Q., Huggins, M.T., Lightner, D.A., Norona, W., and McDonagh, A.F., J. Am. Chem. Soc., 1999, vol. 121, p. 9253. https://doi.org/10.1021/ja991814m
Chen, J., Huang, P.-Q., and Queneau, Y., J. Org. Chem., 2009, vol. 74, p. 7457. https://doi.org/10.1021/jo901557h
Omura, S., Fujimoto, T., Otoguro, K., Matsuzaki, K., Moriguchi, R., Tanaka, H., and Sasaki, Y., J. Antibiot., 1991, vol. 44, p. 113. https://doi.org/10.7164/antibiotics.44.113
Omura, S., Sasaki, Y., and Iwai, Y., J. Antibiot., 1995, vol. 48, p. 535. https://doi.org/10.7164/antibiotics.48.535
Singh, S.B., Goetz, M.A., Jones, E.T., Bills, G.F., Giacobbe, R.A., Herranz, L., Stevens-Miles, S., and Williams Jr, D.L., J. Org. Chem., 1995, vol. 60, p. 7040. https://doi.org/10.1021/jo00126a071
He, H., Yang, H.Y., Bigelis, R., Solum, E.H., Greenstein, M., and Carter, G.T., Tetrahedron Lett., 2002, vol. 43, p. 1633. https://doi.org/10.1016/s0040-4039(02)00099-0
Shiozawa, H. and Takahashi, S., J. Antibiot., 1994, vol. 47, p. 851. https://doi.org/10.7164/antibiotics.47.851
Clark, A.J., Dell, C.P., McDonagh, J.M., Geden, J., and Mawdsley, P., Org. Lett., 2003, vol. 5, p. 2063. https://doi.org/10.1021/ol030045f
Feling, R.H., Buchanan, G.O., Mincer, T.J., Kauffman, C.A., Jensen, P.R., and Fenical, W., Angew. Chem., Int. Ed., 2003, vol. 42, p. 355. https://doi.org/10.1002/anie.200390115
Asami, Y., Kakeya, H., Onose, R., Yoshida, A., Matsuzaki, H., and Osada, H., Org. Lett., 2002, vol. 4, p. 2845. https://doi.org/10.1021/ol020104
Wiedeman, P.E. and Trevillyan, J., Curr. Opin. Invest. Drugs, 2003, vol. 4, p. 412.
Shiraki, R., Sumino, A., Tadano, K.-i., and Ogawa, S., Tetrahedron Lett., 1995, vol. 36, p. 5551. https://doi.org/10.1016/S0040-4039(01)93797-9
Kawasuji, T., Fuji, M., Yoshinaga, T., Sato, A., Fujiwara, T., and Kiyama, R., Bioorg. Med. Chem., 2007, vol. 15, p. 5487. https://doi.org/10.1016/j.bmc.2007.05.052
Lampe, J.W., Chou, Y.L., Hanna, R.G., Di Meo, S.V., Erhardt, P.W., Hagedorn III, A.A., Ingebretsen, W.R., and Cantor, E., J. Med. Chem.,1993, vol. 36, p. 1041. https://doi.org/10.1021/jm00060a012
Peifer, C., Selig, R., Kinkel, K., Ott, D., Totzke, F., Schachtele, C., Heidenreich, R., Röcken, M., Schollmeyer, D., and Laufer, S., J. Med. Chem., 2008, vol. 51, p. 3814. https://doi.org/10.1021/jm8001185
Kakeya, H., Onozawa, C., Sato, M., Arai, K., and Osada, H., J. Med. Chem., 1997, vol. 40, p. 391. https://doi.org/10.1021/jm960719a
Fischer, R., Abmann, L., Lehr, S., Drewes, M.W., Feucht, D., Malsam, O., Bojack, G., Auler, T., Hills, M.J., and Kehne, H., US Patent no. 7420062, 2008.
Adam, W. and Zhang, A., Eur. J. Org. Chem., 2004, vol. 2004, p. 147. https://doi.org/10.1515/ncrs-2016-0079
Raghuraman, A., Ko, E., Perez, L.M., Ioerger, T.R., and Burgess, K., J. Am. Chem. Soc., 2011, vol. 133, p. 12350. https://doi.org/10.1021/ja2033734
Wrobel, M.N and Margaretha, P., Chem. Commun., 1998, p. 541. https://doi.org/10.1039/A708365C
Mizushina, Y., Kobayashi, S., Kuramochi, K., Nagata, S., Sugawara, F., and Sakaguchi, K., Biochem. Biophys. Res. Commun., 2000, vol. 273, p. 784. https://doi.org/10.1006/bbrc.2000.3007
Zhu, Q., Gao, L., Chen, Z., Zheng, S., Shu, H., Li, J., Jiang, H., and Liu, S., Eur. J. Med. Chem., 2012, vol. 54, p. 232. https://doi.org/10.1016/j.ejmech.2012.05.001
Li, B., Lyle, M.P., Chen, G., Li, J., Hu, K., Tang, L., Alaoui-Jamali, M.A., and Webster, J., Bioorg. Med. Chem., 2007, vol. 15, p. 4601. https://doi.org/10.1016/j.bmc.2007.04.017
Borthwick, A.D., Crame, A.J., Ertl, P.F., Exall, A.M., Haley, T.M., Hart, G.J., Mason, A.M., Pennell, A.M., Singh, O.M., and Weingarten, G.G., Eur. J. Med. Chem., 2002, vol. 12, p. 1719. https://doi.org/10.1016/s0960-894x(02)00294-9
Alp, C., Ekinci, D., Gültekin, M.S., Sentürk, M., Sahin, E., and Küfrevioglu, Ö.I., Bioorg. Med. Chem., 2010, vol. 18, p. 4468. https://doi.org/10.1016/j.bmc.2010.04.072
Demir, A.S., Aydogan, F., and Akhmedov, I.M., Tetrahedron: Asymmetry, 2002, vol. 13, p. 601. https://doi.org/10.1016/S0957-4166(02)00140-4
Reddy, T.R., Li, C., Guo, X., Myrvang, H.K., Fischer, P.M., and Dekker, L.V., J. Med. Chem., 2011, vol. 54, p. 2080. https://doi.org/10.1021/jm101212e
Dwoskin, L.P., Teng, L., Buxton, S.T., and Crooks, P.A., J. Pharmacol. Exp. Ther., 1999, vol. 288, p. 905.
Patsalos, P.N., Epilepsia, 2005, vol. 46, p. 140. https://doi.org/10.1111/j.1528-1167.2005.00326.x
Gao, Y., Shirai, M., and Sato, F., Tetrahedron Lett., 1997, vol. 38, p. 6849. https://doi.org/10.1016/S0040-4039(98)01494-4
Manzer, L.E., US Patent no. 6743819, 2004.
Radic, D. and Gargallo, L., Macromolecules, 1997, vol. 30, p. 817. https://doi.org/10.1021/ma960956n
Vijayasekaran, S., Chirila, T.V., Hong, Y., Tahija, S.G., Dalton, P.D., Constable, I.J., and McAllister, I.L. J. Biomater. Sci. Polym. Ed., 1996, vol. 7, p. 685. https://doi.org/10.1163/156856296X00453
Pinza, M., Farina, C., Cerri, A., Pfeiffer, U., Riccaboni, M.T., Banfi, S., Biagetti, R., Pozzi, O., Magnani, M., and Dorigotti, L., J. Med. Chem., 1993, vol. 36, p. 4214. https://doi.org/10.1021/jm00078a011
Liu, X., Xu, Y., Wu, Z., and Chen, H., Macromol. Biosci., 2013, vol. 13, p. 147. https://doi.org/10.1002/mabi.201200269
Wienk, I., Meuleman, E., Borneman, Z., Van Den Boomgaard, T., and Smolders, C., J. Polym. Sci., Part A: Polym. Chem., 1995, vol. 33, p. 49. https://doi.org/10.1002/pola.1995.080330105
Jiang, L.-J., Lv, S.-M., Cheng, C., Dong, L., Li, Y., and Yin, S.-F., Chem. Nat. Compd., 2015, vol. 51, p. 121. https://doi.org/10.1007/s10600-015-1216-9
Ahankar, H., Ramazani, A., Slepokura, K., Lis, T., and Joo, S.W., Green Chem., 2016, vol. 18, p. 3582. https://doi.org/10.1039/c6gc00157b
Fujita, M., Kitagawa, O., Yamada, Y., Izawa, H., Hasegawa, H., and Taguchi, T., J. Org. Chem., 2000, vol. 65, p. 1108. https://doi.org/10.1021/jo991578y
Zhang, L., Tan, Y., Wang, N.-X., Wu, Q.-Y., Xi, Z., and Yang, G.-F., Bioorg. Med. Chem., 2010, vol. 18, p. 7948. https://doi.org/10.1016/j.bmc.2010.09.036
Cuiper, A.D., Kouwijzer, M.L., Grootenhuis, P.D., Kellogg, R.M., and Feringa, B.L., J. Org. Chem., 1999, vol. 64, p. 9529. https://doi.org/10.1021/jo051646i
Zhu, Q., Jiang, H., Li, J., Liu, S., Xia, C., and Zhang, M., J. Comb. Chem., 2009, vol. 11, p. 685. https://doi.org/10.1021/cc900046f
Khan, A.T., Ghosh, A., and Khan, M.M., Tetrahedron Lett., 2012, vol. 53, p. 2622. https://doi.org/10.1016/j.tetlet.2012.03.046
Vidal, J.D., Climent, M.J., Corma, A., Concepcion, D.P., and Iborra, S., ChemSusChem, 2016, vol. 10, p. 119. https://doi.org/10.1002/cssc.201601333
Aginagalde, M., Bello, T., Masdeu, C., Vara, Y., Arrieta, A., and Cossío, F.P., J. Org. Chem., 2010, vol. 75, p. 7435. https://doi.org/10.1021/jo101388x
Rigo, B., Lespagnol, C., and Pauly, M., J. Heterocycl. Chem., 1986, vol. 23, p. 183. https://doi.org/10.1002/jhet.5570230137
Rosse, G., ACS Med. Chem. Lett., 2016, vol. 7, p. 15. https://doi.org/10.1021/acsmedchemlett.5b00493
Brine, G.A. and Boldt, K.G., J. Pharm. Sci., 1983, vol. 72, p. 700. https://doi.org/10.1002/jps.2600720626
Hawkins, J.E. and Sarett, L.H., Clin. Chim. Acta, 1957, vol. 2, p. 481. https://doi.org/10.1016/0009-8981(57)90049-9
Kangani, M., Maghsoodlou, M.-T., and Hazeri, N., Chin. Chem. Lett., 2016, vol. 27, p. 66. https://doi.org/10.1016/j.cclet.2015.07.025
Wong, Y.-C., Parthasarathy, K., and Cheng, C.-H., J. Am. Chem. Soc., 2009, vol. 131, p. 18252. https://doi.org/10.1021/ja9088296
Püschl, A., Boesen, T., Zuccarello, G., Dahl, O., Pitsch, S., and Nielsen, P.E., J. Org. Chem., 2001, vol. 66, p. 707. https://doi.org/10.1021/jo001000k
Burgess, L.E. and Meyers, A., J. Org. Chem., 1992, vol. 57, p. 1656. https://doi.org/10.1021/Jo00032A012
Anaraki-Ardakani, H., Noei, M., and Tabarzad, A., Chin. Chem. Lett., 2012, vol. 23, p. 45. https://doi.org/10.1016/j.cclet.2011.09.010
Schmidt, U. and Geiger, F., Angew. Chem., Int. Ed. Engl., 1962, vol. 1, p. 265. https://doi.org/10.1002/anie.196202652
Buchi, G. and Lukas, G., J. Am. Chem. Soc., 1964, vol. 86, p. 5654. https://doi.org/10.1021/ja01078a049
Gao, H., Sun, J., and Yan, C.-G., Tetrahedron, 2013, vol. 69, p. 589. https://doi.org/10.1016/j.tet.2012.11.018
Rana, S., Brown, M., Dutta, A., Bhaumik, A., and Mukhopadhyay, C., Tetrahedron Lett., 2013, vol. 54, p. 1371. https://doi.org/10.1016/j.tetlet.2012.12.109
Kangani, M., Hazeri, N., and Maghsoodlou, M.-T., J. Saudi Chem. Soc., 2017, vol. 21, p. 160. https://doi.org/10.1016/j.jscs.2015.03.002
Donohoe, T.J., O’Riordan, T.J., Peifer, M., Jones, C.R., and Miles, T.J., Org. Lett., 2012, vol. 14, no. 21, p. 5460. https://doi.org/10.1021/ol302541j
Husain, A., Alam, M.M., and Siddiqui, N., J. Serb. Chem. Soc., 2009, vol. 74, p. 103. https://doi.org/10.2298/JSC091127003K
Ahmad, A., Husain, A., Khan, S.A., Mujeeb, M., and Bhandari, A., J. Saudi Chem. Soc., 2015, vol. 19, p. 340. https://doi.org/10.1016/j.jscs.2014.05.007
Kandile, N.G., Zaky, H.T., Saleh, Y.G., and Ahmed, N.A., J. Enzyme Inhib. Med. Chem., 2013, vol. 28, p. 853. https://doi.org/10.3109/14756366.2012.689298
Husain, A., Alam, M.M., Shaharyar, M., and Lal, S., J. Enzyme Inhib. Med. Chem., 2010, vol. 25, p. 54. https://doi.org/10.3109/14756360902940860
Khokra, S.L., Jyoti, Chetan, Kaushik, P., Alam, M.M., Zaman, M.S., Ahmad, A., Khan, S.A., and Husain, A., Saudi Pharm. J., 2016, vol. 24, p. 705. https://doi.org/10.1016/j.jsps.2015.05.002
El-Shehry, M.F., Abu-Zied, K.M., Ewies, E.F., Awad, S.M., and Mohram, M.E., Pharma Chem., 2013, vol. 5, p. 318.
Husain, A., Khan, M.S.Y., Hasan, S.M., and Alam, M.M., Eur. J. Med. Chem., 2005, vol. 40, p. 1394. https://doi.org/10.1016/j.ejmech.2005.03.012
Husain, A., Alam, M.M., Hasan, S.M., and Yar, M.S., Acta Pol. Pharm., 2009, vol. 66, p. 173.
Phaechamud, T., Mahadlek, J., Charoenteeraboon, J., and Choopun, S., Indian J. Pharm. Sci., 2012, vol. 74, p. 498. https://doi.org/10.4103/0250-474X.110574
Sutariya, S.D., Parmar, K.A., and Kharadi, G.J., Chem. Sin., 2012, vol. 3, p. 854.
Hemalatha, P., Veeraiah, M., Kumar, S.P., Vanasuya, K., Manju, M., and Naika, R., Indian J. Adv. Chem Sci., 2014, vol. 2, p. 50.
Ramesh, K., Gundampati, R.K., Singh, S., Mitra, K., Shukla, A., Jagannadham, M.V., Chattopadhyay, D., Misra, N., and Ray, B., RSC Adv., 2016, vol. 6, p. 25864. https://doi.org/10.1039/C5RA23239B
El-Mohdy, H.A. and Ghanem, S., J. Polym. Res., 2009, vol. 16, p. 1. https://doi.org/10.1007/s10965-008-9196-0
Kim, S.-K., Xie, Z.-F., Jun, C.-S., Kwon, T.-O., Ryu, S.-R., and Chai, K.-Y., Bull. Korean Chem. Soc., 2007, vol. 28, p. 2319. https://doi.org/10.5012/bkcs.2007.28.12.2319
Suzuki, S., Hosoe, T., Nozawa, K., Kawai, K.-I., Yaguchi, T., and Udagawa, S.-I., J. Nat. Prod., 2000, vol. 63, p. 768. https://doi.org/10.1021/np990371x
West, R.R., Ness, J.V., Varming, A.-M., Rassing, B., Biggs, S., Gasper, S., Mckernan, P.A., and Piggott, J., J. Antibiot., 1996, vol. 49, p. 967. https://doi.org/10.7164/antibiotics.49.967
Matviiuk, T., Madacki, J., Mori, G., Orena, B.S., Menendez, C., Kysil, A., André-Barrès, C., Rodriguez, F., Korduláková, J., Mallet-Ladeira, S., Voitenko, Z., Pasca, M.R., Lherbet, C., and Baltas, M., Eur. J. Med. Chem., 2016, vol. 123, p. 462. https://doi.org/10.1016/j.ejmech.2016.07.028
Ignatova, M., Manolova, N., and Rashkov, I., Eur. Polym. J., 2007, vol. 43, p. 1112. https://doi.org/10.1016/j.eurpolymj.2007.01.012
Koz’minykh, V.O., Igidov, N.M., Zykova, S.S., Kolla, V.E., Shuklina, N.S., and Odegova, T.F., Pharm. Chem. J., 2002, vol. 36, p. 188. https://doi.org/10.1023/A:1019832621371
Lattmann, E., Russell, S., Schwalbe, C., Shortt, A., Balaram, P., Theochari, E., Alharbi, M., Narayanan, R., and Lattmann, P., MedChemComm, 2016, vol. 7, p. 1138. https://doi.org/10.1039/C6MD00052E
Abou-Elmagd, W.S., EL-Ziaty, A.K., Elzahar, M.I., Ramadan, S.K., and Hashem, A.I., Synth. Commun., 2016, vol. 46, p. 1197. https://doi.org/10.1080/00397911.2016
Olla, S., Manetti, F., Crespan, E., Maga, G., Angelucci, A., Schenone, S., Bologna, M., and Botta, M., Bioorg. Med. Chem. Lett., 2009, vol. 19, p. 1512. https://doi.org/10.1016/j.bmcl.2009.01.005
Saillenfait, A., Marquet, F., Sabaté, J., Ndiaye, D., and Lambert-Xolin, A., Regul. Toxicol. Pharmacol., 2016, vol. 81, p. 275. https://doi.org/10.1016/j.yrtph.2016.09.011
Qin, Z., Huang, S., Yu, Y., and Deng, H., Mar. Drugs, 2013, vol. 11, p. 3970. https://doi.org/10.3390/md11103970
Nagle, D., Camacho, F., and Paul, V., Mar. Biol., 1998, vol. 132, p. 267. https://doi.org/10.1007/s002270050392
Paul, V.J. and Ritson-Williams, R., Nat. Prod. Rep., 2008, vol. 25, p.662. https://doi.org/10.1039/B702742G
Burja, A.M., Banaigs, B., Abou-Mansour, E., Burgess, J.G., and Wright, P.C., Tetrahedron, 2001, vol. 57, p. 9347. https://doi.org/10.1016/S0040-4020(01)00931-0
Edwards, D.J., Marquez, B.L., Nogle, L.M., McPhail, K., Goeger, D.E., Roberts, M.A., and Gerwick, W.H., Chem. Biol., 2004, vol. 11, p. 817. https://doi.org/10.1016/j.chembiol.2004.03.030
Ali, Y., Alam, M.S., Hamid, H., Husain, A., Shafi, S., Dhulap, A., Hussain, F., Bano, S., Kharbanda, C., and Nazreen, S., Chem. Biol. Drug Des., 2015, vol. 86, p. 619. https://doi.org/10.1111/cbdd.12522
Alam, M.M., Husain, A., Hasan, S.M., Suruchi, and Anwer, T., Eur. J. Med. Chem., 2009, vol. 44, no. 6, p. 2636. https://doi.org/10.1016/j.ejmech.2008.10.030
Bunnelle, E.M., Smith, C.R., Lee, S.K., Singaram, S.W., Rhodes, A.J., and Sarpong, R., Tetrahedron, 2008, vol. 64, p. 7008. https://doi.org/10.1016/j.tet.2008.02.103
Micheli, F., Pasquarello, A., Tedesco, G., Hamprecht, D., Bonanomi, G., Checchia, A., JaxaChamiec, A., Damiani, F., Davalli, S., Donati, D., Gallotti, C., Petrone, M., Rinaldi, M., Riley, G., Terreni, S., and Wood, M., Bioorg. Med. Chem. Lett., 2006, vol. 16, p. 3906. https://doi.org/10.1016/j.bmcl.2006.05.034
Kaushik-Basu, N., Ratmanova, N.K., Manvar, D., Belov, D.S., Cevik, O., Basu, A., Yerukhimovich, M.M., Lukyanenko, E.R., Andreev, I.A., Belov, G.M., Manfroni, G., Cecchetti, V., Frick, D.N., Kurkin, A.V., Altieri, A., and Barreca, M.L., Eur. J. Med. Chem., 2016, vol. 122, p. 319. https://doi.org/10.1016/j.ejmech.2016.06.041
Bertram, J., Schettgen, T., and Kraus, T., J. Chromatogr. B: Biomed. Sci. Appl., 2016, vol. 1033, p. 321. https://doi.org/10.1016/j.jchromb.2016.08.026
Jouyban, A., Fakhree, M.A.A., and Shayanfar, A., J. Pharm. Pharm. Sci., 2010, vol. 13, p. 524. https://doi.org/10.18433/j3p306
Strickley, R.G., Pharm. Res., 2004, vol. 21, p. 201. https://doi.org/10.1023/b:pham.0000016235.32639.23
Xu, W.-L., Mao, F., Zhao, H.-K., Wang, Y.-Q., and Wang, J., J. Chem. Eng. Data, 2007, vol. 52, p. 553. https://doi.org/10.1021/je060454x
Zheng, W.-W., Zhao, L., Wei, Y.-M., Ye, Y., and Xiao, S.-H., Chem. Pharm. Bull., 2010, vol. 58, p. 1015. https://doi.org/10.1248/cpb.58.1015
Yang, P., Ding, X., Gao, J., and Gao, S., Acad. J. Second Mil. Med. Univ., 2007, vol. 11, p. 26.
Fujii, M., Bouno, M., Fujita, S., Yoshida, M., Watanabe, Y., and Matsumoto, M., Biol. Pharm. Bull., 2000, vol. 23, p. 1341. https://doi.org/10.1248/bpb.23.1341
Yerramsetty, K., Neely, B., Madihally, S., and Gasem, K., Int. J. Pharm., 2010, vol. 388, p. 13. https://doi.org/10.1016/j.ijpharm.2009.12.028
Koizumi, A., Fujii, M., Kondoh, M., and Watanabe, Y., Eur. J. Pharm. Biopharm., 2004, vol. 57, p. 473. https://doi.org/10.1016/j.ejpb.2003.12.006
Babu, R.J. amd Pandit, J.K., Drug Delivery, 2005, vol. 12, p. 165. https://doi.org/10.1080/10717540590931936
Kikwai, L., Babu, R.J., Prado, R., Kolot, A., Armstrong, C.A., Ansel, J.C., and Singh, M., AAPS PharmSciTech, 2005, vol. 6, p. E565. https://doi.org/10.1208/pt060471
Bhatia, K.S. and Singh, J., Drug Dev. Ind. Pharm., 1997, vol. 23, p. 1111. https://doi.org/10.3109/03639049709150501
Akhter, S.A. and Barry, B.W., J. Pharm. Pharmacol., 1985, vol. 37, p. 27. https://doi.org/10.1111/j.2042-7158.1985.tb04926.x
Sugibayashi, K., Sakanoue, C., and Morimoto, Y., Sel. Cancer Ther., 1989, vol. 5, p. 119. https://doi.org/10.1089/sct.1989.5.119
Hosseinzadeh, Z., Ramazani, A., Hosseinzadeh, K., Razzaghi-Asl, N., and Gouranlou, F., Curr. Org. Synth., 2018, vol. 15, p. 166. https://doi.org/10.2174/1570179414666170908165445
Zhang, Y., Zhou, X., Xie, Y., Greenberg, M.M., Xi, Z., and Zhou, C., J. Am. Chem. Soc., 2017, vol. 139, p. 6146. https://doi.org/10.1021/jacs.7b00670
ACKNOWLEDGMENTS
The authors thank the Glocal School of Pharmacy, Glocal University, Mirzapur Pole, Saharanpur, Uttar Pradesh, India, and Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Northern Border University, Rafha, Saudi Arabia, for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Asif, M., Alghamdi, S. An Overview on Biological Importance of Pyrrolone and Pyrrolidinone Derivatives as Promising Scaffolds. Russ J Org Chem 57, 1700–1718 (2021). https://doi.org/10.1134/S1070428021100201
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021100201