Abstract
To obtain new lead compounds with potent antimicrobial and antioxidant activities, new indole analogs containing various heterocyclic moieties were synthesized by a rapid, facile, eco-friendly, cost-effective, and efficient method under microwave irradiation. Their structures were determined on the basis of their spectral and elemental analysis data. The compounds were evaluated for their in vitro antimicrobial and antioxidant activities. Some of the compounds revealed good antimicrobial and antioxidant activities. The structure–activity relationship (SAR) study revealed that the most active are compounds containing a substituent at the 5-position of indole.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Green chemistry (GC) addresses our future challenges in working with chemical processes and products by using methods that can maximize the desired products and minimize by-products, simplify operations in chemical production, and use environmentally benign solvents. The concept of green chemistry has emerged in the early 1990s and is now widely used to meet the major scientific challenges concerned with protecting the human health and the environment while simultaneously achieving commercial feasibility. Non-classical methods following the GC principles reduce or even eradicate the production of harmful substances and increase product yield. Microwave (MW)-assisted synthesis is one of the important GC techniques used during the recent years. The ability of MW-assisted reactions to rapidly synthesize organic compounds is of major benefit for library generation. Moreover, MW-assisted synthesis allows modifications of selectivity (chemo-, regio-, and stereoselectivity) and the use of solvent-free and catalyst-free conditions [1–4].
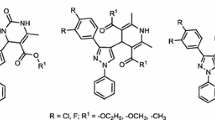
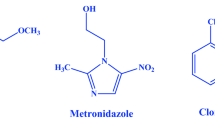
In the past few decades, the synthesis of heterocyclic compounds has played a significant role in medicinal chemistry, and many advances have been achieved in the practical aspects, including novel synthetic strategies and methods and analytical techniques. Benzimidazole derivatives are associated with various types of pharmacological properties. Benzimidazole is one of the most bioactive heterocyclic compounds that exhibit a wide range of biological activities. Specifically, benzimidazole nucleus is a constituent of vitamin B12 [5] and many other pharmacologically active compounds [6–9]. Some examples of clinically approved drugs are shown in Fig. 1.
It is well known that indole moiety is probably the most widely spread nitrogen heterocycle in nature and that it is very important for its medicinal and biological aspects. Indole derivatives have been found to possess pharmacological and chemotherapeutic properties such as anticancer, antidiabetic, anti-inflammatory, antimalarial, antibacterial, antifungal, antiviral, etc. [1–4, 10–15]. Thiazolidin-4-ones and azetidinones also play an important role in medicinal chemistry [2]; several analogs of these compounds have been extensively studied due to their ready accessibility, diverse chemical reactivity, and broad spectrum of biological properties.
In view of the literature reports and the wide application of benzimidazole drugs for the treatment of various diseases, herein we describe the microwave-assisted synthesis of benzimidazole derivatives containing an indole fragment in combination with thiazolidinone, triazole, azetidinone, or thioether moiety with the goal of obtaining compounds with better biological activities.
RESULTS AND DISCUSSION
In the present study, we were interested in using ecofriendly reagents and methods and avoiding the use of organic solvents to meet the GC principles. The target indolyl benzimidazole analogs were synthesized in two steps as outlined in Scheme 1. In the first step, hydrazones 3a–3d were prepared by reacting 2,5-substituted 1H-indole-3-carbaldehydes 1a–1d and an equimolar amount of 2-hydrazinyl-1H-benzimidazole (2) in ethanol in the presence of glacial acetic acid. Hydrazones 3a–3d were cyclized with thioglycolic acid in the presence of anhydrous zinc chloride to produce thiazolidin-4-one derivatives 4a–4d. Cyclocondensation of 3a–3d in acetic anhydride under reflux afforded [1,2,4]triazolo[4,3-b]benzimidazole derivatives 5a–5d. Azetidinone derivatives 6a–6d were obtained by cyclization of 3a–3d with chloroacetyl chloride in 1,4-dioxane. Finally, treatment of 3a–3d with 1H-benzimidazole-2-thiol furnished thioether derivatives 7a–7d.
All these reactions were carried out under both conventional heating conditions and microwave irradiation. The conventional syntheses gave the corresponding products in low yields with moderate purity and suffered from many disadvantages such as the use of solvent, long reaction time, and lengthy workup process. In contrast, MW irradiation provided a rapid, economical, and environmentally benign procedure, and the target compounds were obtained in excellent yields with high purity (Table 1).
All compounds 3–7 are stable solids soluble in DMSO in room temperature. Their structure was confirmed by FT-IR, 1H and 13C NMR, and mass spectra. The IR spectrum of 3a showed absorption peaks at 3423, 3238, and 3106 cm–1 due to indole, imidazole, and hydrazine N–H stretchings, respectively, a peak at 2926 cm–1 due to aromatic C–H bond vibrations, and a peak at 1704 cm–1 due to CH=N imine bond. Other peaks at 1457 and 509 cm–1 were assigned to C=N and C–Cl bonds, respectively. The 1H NMR spectrum of 3a showed a singlet at δ 2.06 ppm (1H, NH), a multiplet at δ 6.55–7.30 ppm (12H, Harom), a singlet at δ 9.66 ppm (NH, imidazole), and a singlet at δ 10.09 ppm (NH, indole). The 13C NMR spectrum of 3a displayed signals at δC 112, 113, 120, 123, 126, 129, 130, 134, and 150 due to aromatic carbons, and the most deshielded imine carbon resonated at δC 185 ppm. The mass spectrum of 3a contained the doublet molecular ion peak at m/z 383/385 (Irel 100/33%) with the intensity ratio (3:1) corresponding to the presence of one chlorine atom in its molecule.
The IR spectrum of 4a showed absorption bands at 3400, 3383, and 3080 cm–1 due to N–H stretchings and a carbonyl band at 1654 cm–1. Other peaks at 1520, 602, and 498 cm–1 were assigned to the C=N, C–Cl, C–S–C bonds, respectively. The 1H NMR spectrum of 4a showed a singlet at δ 2.57 ppm (1H, NH), a singlet at δ 4.37 ppm (2H, CH2) due to methylene protons of the thiazolidine ring, a singlet at δ 5.99 ppm (1H) due to the SCHN proton of thiazolidinone, a multiplet between δ 6.98–7.42 (12H, Harom), and singlets at δ 8.65 and 9.89 ppm due to imidazole and indole NH protons, respectively. The 13C NMR spectrum of 4a showed signals at δC 107, 112, 113, 114, 115, 116, 120, 123, 121, 126, 129, 130, 134, 135, 149, and 150 ppm due to aromatic carbons and downfield signals at δC 154 (C2, thiazolidine) and 185 ppm (C=O). The mass spectrum of 4a showed the doublet molecular ion peak at m/z 459/461 (Irel 45/15%).
The spectral data for the other compounds were consistent with their structure. In particular, in the 13C NMR spectrum of 5a, the most deshielded carbons attached to three nitrogen atoms appeared at δC 149 and 151 ppm. The IR spectrum of 6a showed a peak at 1684 cm–1 due to carbonyl group and two peaks at 730 and 704 cm–1 due to C–Cl stretching vibrations. The 1H NMR spectrum of 6a displayed signals at δ 4.59 and 5.46 ppm for protons in the azetidinone ring, and the corresponding carbon signals were observed in the 13C NMR spectrum at δC 58 and 66 ppm together with the carbonyl carbon signal at δC 181 ppm. The mass spectrum of 6a contained the molecular ion triplet at m/z 461/463/465 with an intensity ratio of 9:6:1, which corresponded to the presence of two chlorine atoms in its molecule. The absorption band at 689 cm–1 in the IR spectrum of 7a was assigned to the C–S bond, and the CH–S fragment of 7a gave signals at δ 4.38 and δC 70 ppm in the NMR spectra.
In vitro antimicrobial activity. The newly synthesized compounds were screened for their in vitro antibacterial activity against Staphylococcus aureus (ATCC 29513), Pseudomonas aeruginosa (MTCC 1688), Escherichia coli (MTCC 723), Klebsiella pneumoniae (NCTC 13368), and Salmonella typhi (ATCC 23564) and antifungal activity against Aspergillus oryzae (MTCC 3567T), Aspergillus terreus (MTCC 1782), Aspergillus niger (MTCC 281), and Aspergillus flavus (MTCC 1973) by the cup-plate method as reported in [10, 11]. All tested compounds showed moderate to high antibacterial activity as compared to the standard drug streptomycin (Table 2). Compounds 4a, 5a, and 6a showed excellent antibacterial activities against S. aureus. Compounds 4a and 6a exhibited a good activity against P. aeruginosa, and compound 4a displayed a good activity against E. coli. On the other hand, evaluation of the antifungal activity (Table 3) revealed a significant activity of 3a against A. niger, good activities of 5a and 7a against A. oryzae, and a good activity of 5a against A. terreus. The other compounds were either moderately active or inactive against the bacterial and fungal strains.
The obtained result showed that derivatives 4a, 5a, and 7a containing a chlorine atom at the 5-position of the indole ring are the most active against different microbial strains at a concentration of 25–100 µg/mL. The role of electron-withdrawing groups in enhancing antibacterial activity has been reported [1, 8].
Antioxidant activity. The free radical scavenging activity of compounds 3–7 was evaluated using DPPH assay as reported in [10, 11]. The results showed (Fig. 2) that compounds 3d, 4d, 5d, 5c, and 6d were the most potent at concentrations of 25, 50, 75, and 100 µg/mL; compounds 5c, 7b, and 7c exhibited good radical scavenging activity at those concentrations, and the other compounds were moderately active.
The reductive ability of the synthesized compounds was assessed by the ferric ion-reducing antioxidant power (FRAP) assay which implies the conversion of Fe3+/ferricyanide complex to the Fe2+/ferrous form [10, 11]. The results (Fig. 3) displayed excellent reducing power ability of compounds 3d and 4d and good reducing power of 5c, 5d, 6c, 6d, 7c, and 7d at concentrations of 25, 50, 75, and 100 µg/mL. The other compounds exhibited moderate reducing ability.
The total antioxidant capacity of the synthesized compounds was evaluated by the phosphomolybdenum method as described in [10, 11]. As seen from Fig. 4, compounds 4a, 5d, 7c, and 7d exhibited excellent total antioxidant capacity at 25, 50, 75, and 100 µg/mL. The other compounds were moderately active.
Structure–activity relationship. The obtained results suggest that halogen (Cl) substitution at the 5-position of the indole ring attached to different heterocycles favors enhanced antimicrobial activity. On the other hand, compounds having no substituent at the indole C5 atom show good antioxidant activity. It is clear that the presence of heteroatoms like N, S, O, and halogens (Cl) provides additional binding interactions inside the active site of enzymes of microorganisms, which may contribute to the activity of the compounds.
EXPERIMENTAL
The chemicals used in this study were purchased from Merck, HiMedia, and SD Fine Chem and were used without further purification. The progress of reactions was monitored by TLC on Silica gel 60 F245 plates (Merck) using ethyl acetate–hexane (5:1 v/v) as eluent; spots were detected by exposure to a UV lamp at λ 254 nm. The melting points were measured in open capillary tubes and are uncorrected. The IR spectra were recorded in KBr on a Perkin Elmer FT-IR spectrometer. The 1H and 13C NMR spectra were recorded on a Bruker spectrometer at 400 and 100 MHz, respectively, using tetramethylsilane as internal standard. The mass spectra were run on a Shimadzu LCMS 2010A instrument. Microwave-assisted reactions were carried out in an ONIDA 20STP21 multimode microwave oven (800 W).
2,5-Substituted indole-3-carbaldehydes 1a–1d were prepared by the Bischler method, followed by Vilsmeier–Haack formylation [16]; 1H-benzimidazole-2-thiol and 2-hydrazinyl-1H-benzimidazole (2) were prepared as reported in [17].
Hydrazones 3a–3d (general procedure). a. Conventional method. A mixture of 1H-indole-3-carbaldehyde 1a–1d (0.01 mol), 2-hydrazinyl-1H-benzimidazole (2), and 4–5 drops of glacial acetic acid in methanol (35 mL) was refluxed on a water bath for 5– 6 h, following the reported procedure [2]. The progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was cooled and poured into ice water. The resulting solid was filtered off, washed with a solution of sodium hydrogen sulfate and then with cold water, dried, and recrystallized from ethanol.
b. Microwave-assisted synthesis. An open borosil glass tube was charged with a mixture of aldehyde 1a–1d (0.01 mol) and 2-hydrazinyl-1H-benzimidazole (2) (0.01 mol) in hot methanol (5 mL) containing 4– 5 drops of glacial acetic acid, and the mixture was irradiated in a MW oven at 90–100°C for 4–5 min according to the reported method [2]. The progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was cooled and poured into ice water, and the resulting solid was filtered off, washed with a solution of sodium hydrogen sulfate and then with cold water, dried, and recrystallized from ethanol.
2-{2-[(5-Chloro-2-phenyl-1H-indol-3-yl)methylidene]hydrazinyl}-1H-benzimidazole (3a). Yield 95%, dark yellow powder, mp 148–149°C. IR spectrum, ν, cm–1: 3423 (NH, indole), 3238 (NH, imidazole), 3106 (NH), 2926 (C–Harom), 1704 (HC=N), 1457(C=N), 509 (C–Cl). 1H NMR spectrum (CDCl3), δ, ppm: 2.06 s (1H, NH), 6.55–7.30 m (12H, Harom), 8.15 s (1H, HC=N), 9.66 s (1H, NH, imidazole), 10.09 s (1H, NH, indole). 13C NMR spectrum (DMSO-d6), δC, ppm: 112, 113, 120, 123, 126, 129, 130, 134, 150 (Carom); 185.5 (HC=N). Mass spectrum: m/z 383/385 (Irel 100/33%) [M]+.
2-{2-[(5-Methyl-2-phenyl-1H-indol-3-yl)methylidene]hydrazinyl}-1H-benzimidazole (3b). Yield 92%, light purple crystals, mp 202–203°C. IR spectrum, ν, cm–1: 3422 (NH, indole), 3200 (NH, imidazole), 3046 (NH), 2849–2904 (CH3), 1753 (HC=N), 1435 (C=N, imidazole). 1H NMR spectrum (CDCl3), δ, ppm: 1.64 s (3H, CH3), 2.19 s (1H, NH), 6.98–7.71 m (12H, Harom), 8.29 s (1H, HC=N), 8.68 s (1H, NH, imidazole), 10.00 s (1H, NH, indole). 13C NMR spectrum (DMSO-d6), δC, ppm: 21.29 (CH3); 110, 111, 115, 118, 119, 120, 122, 123, 124, 126, 127, 128, 129, 131, 133, 134, 137, 142 (Carom); 153 (HC=N). Mass spectrum: m/z 365 [M]+.
2-{2-[(2-Phenyl-1H-indol-3-yl)methylidene]hydrazinyl}-1H-benzimidazole (3c). Yield 90%, light purple crystals, mp 179–180°C. IR spectrum, ν, cm–1: 3243 (NH, indole), 3106 (NH, imidazole), 3016 (NH), 2915 (C–Harom), 1720 (HC=N), 1457(C=N, imidazole). 1H NMR spectrum (CDCl3), δ, ppm: 2.02 s (1H, NH), 7.22–7.43 m (13H, Harom), 7.98 s (1H, HC=N), 9.06 s (1H, NH, imidazole), 10.06 s (1H, NH, indole). Mass spectrrum: m/z 351 [M]+.
2-{2-[(1H-Indol-3-yl)methylidene]hydrazinyl}-1H-benzimidazole (3d). Yield 85%, dark brown crystals, mp 210–211°C. IR spectrum, ν, cm–1: 3408 (NH, indole), 3286 (NH, imidazole), 3178 (NH), 1758 (HC=N), 1567 (C=N, imidazole). 1H NMR spectrum (CDCl3), δ ppm: 2.16 s (1H, NH), 6.94–7.40 m (9H, Harom), 7.96 s (1H, HC=N), 9.17 s (1H, NH, imidazole), 10.37 s (1H, NH, indole). Mass spectrum: m/z 275 [M]+.
Thiazolidin-4-one derivatives 4a–4d (general procedure). a. Conventional method. A mixture of hydrazone 3a–3d (0.01 mol), thioglycolic acid (0.7 mL, 0.01 mol), and a pinch of anhydrous zinc chloride in dry DMF (15 mL) was refluxed for 10–12 h [2]. After completion of the reaction (TLC), the mixture was cooled and neutralized with a 10% aqueous solution of sodium hydrogen carbonate. The solid product was filtered off, washed with water, dried, and recrystallized from ethanol.
b. Microwave-assisted synthesis. An open borosil glass tube was charged with a mixture of 3a–3d (0.01 mol), thioglycolic acid (0.7 mL, 0.01 mol), 50 mg of anhydrous zinc chloride, and DMF (5 mL), and the mixture was subjected to MW irradiation at 160–170°C for 5–6 min [2]. After completion of the reaction (TLC), the mixture was cooled and neutralized with a 10% aqueous solution of sodium hydrogen carbonate. The solid product was filtered off, washed with water, dried, and recrystallized from ethanol.
3-[(1H-Benzimidazol-2-yl)amino]-2-(5-chloro-2-phenyl-1H-indol-3-yl)thiazolidin-4-one (4a). Yield 92%, dark yellow crystals, mp 151–152°C. IR spectrum, ν, cm–1: 3440 (NH, indole), 3383 (NH, imidazole), 3080 (NH), 1654 (C=O), 1520 (C=N), 602 (C–Cl), and 498 (C–S–C). 1H NMR spectrum (CDCl3), δ, ppm: 2.57 s 1H (NH), 4.37 s (2H, CH2), 5.99 s (1H, SCHN), 6.98–7.42 m (12H, Harom), 8.65 s (1H, NH, imidazole), 9.89 s (1H, NH, indole). 13C NMR spectrum (DMSO-d6), δC, ppm: 38.04 and 40.09 (CH2, SCHN); 107, 112, 113, 114, 115, 116, 120, 123, 121, 126, 129, 130, 134, 135, 149, 150 (CCl), 185.5 (C=O). Mass spectrum: m/z 459/461 [M]+.
3-[(1H-Benzimidazol-2-yl)amino]-2-(5-methyl-2-phenyl-1H-indol-3-yl)thiazolidin-4-one (4b). Yield 90%, light gray powder, mp 205–206°C. IR spectrum, ν, cm–1: 3413 (NH, indole), 3218 (NH, imidazole), 3171 (NH), 2849–2920 (CH3), 1698 (C=O), 1435 (C=N), 547 (C–S–C). 1H NMR spectrum (CDCl3), δ, ppm: 1.47 s (3H, CH3), 2.48 s (1H, NH), 4.37 s (2H, CH2), 5.89 s (1H, SCHN), 6.97–7.80 m (12H, Harom), 8.64 s (1H, NH, imidazole), 10.27 s (1H, NH, indole). 13C NMR spectrum (DMSO-d6), δC, ppm: 21.30 (CH3); 38.04 and 40.10 (CH2, SCHN); 111, 113, 120, 125, 126, 128, 129, 131, 134 (Carom); 149 (C=O). Mass spectrum: m/z 439 [M]+.
3-[(1H-Benzimidazol-2-yl)amino]-2-(2-phenyl-1H-indol-3-yl)thiazolidin-4-one (4c). Yield 88%, white shining crystals, mp 214–215°C. IR spectrum, ν, cm–1: 3411 (NH, indole), 3393 (NH, imidazole), 3000 (NH), 1600 (C=O), 1531 (C=N), 747 (C–S–C). 1H NMR spectrum (CDCl3), δ, ppm: 2.16 s (1H, NH), 4.31 s (2H, CH2), 6.19 s (1H, SCHN), 6.94–7.39 m (13H, Harom), 7.98 s (1H, NH, imidazole), 10.38 s (1H, NH, indole). Mass spectrum: m/z 425 [M]+.
3-[(1H-Benzimidazol-2-yl)amino]-2-(1H-indol-3-yl)thiazolidin-4-one (4d). Yield 80%, dark brown powder, mp 207–208°C. IR spectrum, ν, cm–1: 3485 (NH, indole), 3192 (NH, imidazole), 3133 (NH), 1634 (C=O), 1567 (C=N), 715 (C–S–C). 1H NMR spectrum (CDCl3), δ, ppm: 2.62 s (1H, NH), 4.36 s (2H, CH2), 6.19 s (1H, SCHN), 7.22–7.43 m (9H, Harom), 7.98 s (1H, NH, imidazole), 10.59 s (1H, NH, indole). Mass spectrum: m/z = 349 [M]+.
[1,2,4]Triazolo[4,3-b]benzimidazoles 5a–5d. a. Conventional method. A mixture of compound 3a–3d (0.01 mol) and acetic anhydride (10 mL) was refluxed for about 3–4 h, following the reported procedure [18]. After completion of the reaction (TLC), the mixture was cooled to room temperature and poured into ice water. The solid product was filtered off, washed thoroughly with cold water, dried, and recrystallized from 1,4-dioxane.
b. Microwave-assisted synthesis. An open borosil glass tube was charged with a mixture of 3a–3d (0.01 mol) and acetic anhydride (10 mL), and the mixture was irradiated in a MW oven at 140–150°C for 2–4 min. After completion of the reaction (TLC), the mixture was cooled to room temperature and poured into ice water, and the solid product was filtered off, washed thoroughly with cold water, dried, and recrystallized from 1,4-dioxane.
3-(5-Chloro-2-phenyl-1H-indol-3-yl)-9H-[1,2,4]triazolo[4,3-b]benzimidazole (5a). Yield 80%, dark brown powder, mp 145–146°C. IR spectrum, ν, cm–1: 3459 (NH, indole), 3369 (NH, imidazole), 1443 (C=N), 591 (C–Cl). 1H NMR spectrum (CDCl3), δ, ppm: 6.86–7.50 m (12H, Harom), 8.40 s (1H, NH, imidazole), 10.02 s (1H, NH, indole). 13C NMR spectrum (DMSO-d6), δC, ppm: 112, 113, 116, 117, 118, 121, 123, 126, 129, 130, 131, 134, 135 (Carom); 149, 151 (C3, C5, triazole). Mass spectrum: m/z 383/385 [M]+.
3-(5-Methyl-2-phenyl-1H-indol-3-yl)-9H-[1,2,4]triazolo[4,3-b]benzimidazole (5b). Yield 75%, light purple powder, mp 181–182°C. IR spectrum, ν, cm–1: 3422 (NH, indole), 3382 (NH, imidazole), 2849–2950 (CH3), 1523 (C=N). 1H NMR spectrum (CDCl3), δ, ppm: 2.00 s (3H, CH3), 6.84–7.49 m (12H, Harom), 8.54 s (1H, NH, imidazole), 9.98 s (1H, NH, indole). 13C NMR spectrum (DMSO-d6), δC, ppm: 21.54 (CH3); 111, 113, 120, 125, 126, 128, 129, 130, 131, 134 (Carom); 148 (CH, triazole). Mass spectrum: m/z 363 [M]+.
3-(2-Phenyl-1H-indol-3-yl)-9H-[1,2,4]triazolo[4,3-b]benzimidazole (5c). Yield 75%, light brown shining crystals, mp 220–221°C. IR spectrum, ν, cm–1: 3448 (NH, indole), 3242 (NH, imidazole), 3022 (C–Harom), 1535 (C=N). 1H NMR spectrum (CDCl3), δ, ppm: 6.93–7.85 m (13H, Harom), 8.15 s (1H, NH, imidazole), 9.12 s (1H, NH, indole). Mass spectrum: m/z 349 [M]+.
3-(1H-Indol-3-yl)-9H-[1,2,4]triazolo[4,3-b]benzimidazole (5d). Yield 70%, dark brown powder, mp 201–202°C. IR spectrum, ν, cm–1: 3348 (NH, indole), 3286 (NH, imidazole), 3152 (C–Harom), 1680 (C=N). 1H NMR spectrum (CDCl3), δ, ppm: 7.23– 7.99 m (9H, Harom), 8.00 s (1H, NH, imidazole), 9.01 s (1H, NH, indole). Mass spectrum: m/z 273 [M]+.
Azitidin-2-one derivatives 6a–6d (general procedure). a. Conventional method. A few drops of triethylamine and chloroacetyl chloride (0.01 mol) were added with stirring at room temperature over a period of about 15 min to a mixture of compound 3a–3d (0.01 mol) and anhydrous benzene (30 mL). The mixture was refluxed for 2–3 h on a water bath, following the reported procedure [19], and the precipitate of triethylamine hydrochloride was filtered off and washed several times with benzene. The filtrate was combined with the washings and concentrated under reduced pressure, and the residue was washed with petroleum ether (bp 40–60°C) to remove unreacted initial compound 3, dried, and recrystallized from 1,4-dioxane.
b. Microwave-assisted synthesis. A few drops of triethylamine and chloroacetyl chloride (0.01 mol) were added with stirring at room temperature over a period of about 15 min to a mixture of compound 3a–3d (0.01 mol) and anhydrous benzene (5 mL). The mixture was transferred into an open borosil glass tube and irradiated in a MW oven at 100–110°C for 2–4 min, the precipitate of triethylamine hydrochloride was filtered off and washed several times with benzene, the filtrate was combined with the washings and concentrated under reduced pressure, and the residue was washed with petroleum ether (bp 40–60°C) to remove unreacted initial compound 3, dried, and recrystallized from 1,4-dioxane.
1-[(1H-Benzimidazol-2-yl)amino]-3-chloro-4-(5-chloro-2-phenyl-1H-indol-3-yl)azetidin-2-one (6a). Yield 90%, light brown crystals, mp 221–223°C. IR spectrum, ν, cm–1: 3312 (NH, indole), 3201 (NH, imidazole), 3060 (NH), 2926 (C–H), 1684 (C=O), 1637 (C=N), 730 (C3–Cl), 704 (C5′–Cl). 1H NMR spectrum (CDCl3), δ, ppm: 2.62 s (1H, NH), 4.59 d (1H, 4-H), 5.46 d (1H, 3-H), 6.17–7.98 m (12H, Harom), 9.35 s (1H, NH, imidazole), 9.85 s (1H, NH, indole). 13C NMR spectrum (DMSO-d6), δC, ppm: 58 (C4), 66 (C3); 108, 109, 121, 122, 124, 125, 126, 128, 129, 131, 132, 133, 134, 135, 136, 137, 138, 145, 147, 149, 164 (Carom); 181 (C=O). Mass spectrum: m/z 461/463/465 (intensity ratio 9:6:1) [M]+.
1-[(1H-Benzimidazol-2-yl)amino]-3-chloro-4-(5-methyl-2-phenyl-1H-indol-3-yl)azetidin-2-one (6b). Yield 88%, light yellow crystals, mp 205–208°C. IR spectrum, ν, cm–1: 3311 (NH, indole), 3250 (NH, imidazole), 3174 (NH), 2952 (C–H), 2830–2890 (CH3), 1661 (C=O), 1544 (C=N), 726 (C–Cl). 1H NMR spectrum (CDCl3), δ, ppm: 1.78 s (3H, CH3), 2.17 s (1H, NH), 4.58 d (1H, 4-H), 5.43 d (1H, 3-H), 6.94–7.98 m (12H, Harom), 8.88 s (1H, NH, imidazole), 10.51 s (1H, NH, indole). 13C NMR spectrum (DMSO-d6), δC, ppm: 21 (CH3), 60 (C4), 66 (C3); 114, 116, 118, 119, 123, 127, 128, 130, 131, 134, 135, 144, 149, 152 (Carom); 180 (C=O). Mass spectrum: m/z 441/443 (3:1) [M]+.
1-[(1H-Benzimidazol-2-yl)amino]-3-chloro-4-(2-phenyl-1H-indol-3-yl)azetidin-2-one (6c). Yield 85%, light yellow crystals, mp 131–133°C. IR spectrum, ν, cm–1: 3309 (NH, indole), 3051 (NH, imidazole), 3000 (NH), 1700 (C=O), 1655 (C=N), 721 (C–Cl). 1H NMR spectrum (CDCl3), δ, ppm: 2.00 s (1H, NH), 3.58 d (1H, 4-H), 4.31 d (1H, 3-H), 6.17–7.98 m (13H, Harom), 9.88 s (1H, NH, imidazole), 10.16 s (1H, NH, indole). Mass spectrum: m/z 427/429 (3:1) [M]+.
1-[(1H-Benzimidazol-2-yl)amino]-3-chloro-4-(1H-indol-3-yl)azetidin-2-one (6d). Yield 78%, light yellow crystals, mp 124–126°C. IR spectrum, ν, cm–1: 3433 (NH, indole), 3119 (NH, imidazole), 3042 (NH), 3005 (C–Harom), 2816–2953 (C–H), 1649 (C=O), 1621 (C=N), 754 (C–Cl). 1H NMR spectrum (CDCl3), δC, ppm: 2.62 s (1H, NH), 3.59 d (1H, 4-H), 4.36 d (1H, C3), 7.22–7.98 m (8H, Harom), 9.22 s (1H, NH, imidazole), 10.59 s (1H, NH, indole). Mass spectrum: m/z 351/353 (3:1) [M]+.
Thioethers 7a–7d (general procedure). a. Conventional method. A mixture of compound 3a–3d (0.01 mol) and 1H-benzimidazole-2-thiol (0.01 mol) was heated at 150–160°C in an oil bath for 1–2 h according to the reported procedure [20]. After completion of the reaction (TLC), the mixture was cooled to room temperature, and the solid product was filtered off, washed with cold ethanol, and recrystallized from 1,4-dioxane.
b. Microwave-assisted synthesis. An open borosil glass tube was charged with a mixture of compound 3a–3d (0.01 mol) and 1H-benzimidazole-2-thiol (0.01 mol), and the mixture was irradiated in a MW oven at 150–160°C for 2–4 min. After completion of the reaction (TLC), the mixture was cooled to room temperature, and the solid product was filtered off, washed with cold ethanol, and recrystallized from 1,4-dioxane.
2-({[2-(1H-Benzimidazol-2-yl)hydrazinyl](5chloro-2-phenyl-1H-indol-3-yl)methyl}sulfanyl)-1H-benzimidazole (7a). Yield 94%, light brown crystals, mp 228–230°C. IR spectrum, ν, cm–1: 3429 (NH, indole), 3295 (NH, imidazole), 2959 (NH), 1661 and 1608 (C=N), 745 (C–Cl), 689 (C–S–C). 1H NMR spectrum (CDCl3), δ, ppm: 2.19 s and 2.64 s (1H each, NHNH), 4.38 s (1H, CH), 6.98–8.28 m (16H, Harom), 8.29 s (1H, NH, imidazole), 8.60 s (1H, NH, indole). 13C NMR spectrum (DMSO-d6), δC, ppm: 70 (CHS); 112, 113, 119, 122, 123, 124, 125, 126, 127, 128, 130, 132, 134, 135, 136, 147, 149, 166, 170 (Carom). Mass spectrum: m/z 535/537 (3:1) [M]+.
2-({[2-(1H-Benzimidazol-2-yl)hydrazinyl](5methyl-2-phenyl-1H-indol-3-yl)methyl}sulfanyl)-1H-benzimidazole (7b). Yield 92%, dark gray crystals, mp 178–180°C. IR spectrum, ν, cm–1: 3264 (NH, indole), 3179 (NH, imidazole), 3058 (NH), 2882–2964 (CH3), 1694 and 1613 (C=N), 685 (C–S–C). 1H NMR spectrum (CDCl3), δ, ppm: 1.62 s (3H, CH3), 2.19 s and 2.64 s (1H each, NHNH), 4.37 s (1H, CHS), 6.86–8.23 m (16H, Harom), 8.25 s (1H, NH, imidazole), 8.40 s (1H, NH, indole). 13C NMR spectrum (DMSO-d6), δC, ppm: 21 (CH3), 66 (CHS); 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 134, 135, 136, 145, 148, 166, 178 (Carom). Mass spectrum: m/z 515 [M]+.
2-({[2-(1H-Benzimidazol-2-yl)hydrazinyl](2-phenyl-1H-indol-3-yl)methyl}sulfanyl)-1H-benzimidazole (7c). Yield 90%, dark gray crystals, mp 174–176°C. IR spectrum, ν, cm–1: 3392 (NH, indole), 3271 and 3183 (NH, imidazole), 3061 (NH), 2965 (CHS), 1694 and 1610 (C=N), 686 (C–S–C). 1H NMR spectrum (CDCl3), δ, ppm: 2.19 s and 2.64 s (1H each, NHNH), 4.37 s (1H, CH), 6.98–8.26 m (17H, Harom), 8.28 s (1H, NH, imidazole), 8.65 s (1H, NH, indole). Mass spectrum: m/z 501 [M]+.
2-({[2-(1H-Benzimidazol-2-yl)hydrazinyl](2-phenyl-1H-indol-3-yl)methyl}sulfanyl)-1H-benzimidazole (7d). Yield 88%, dark brown crystals, mp 166–168°C. IR spectrum, ν, cm–1: 3364 (NH, indole), 3176 (NH, imidazole), 3059 (NH), 1610 and 1540 (C=N), 688 (C–S–C). 1H NMR spectrum (CDCl3), δ, ppm: 2.06 s and 2.52 s (1H each, NHNH), 4.30 s (1H, CHS), 6.55–7.97 m (13H, Harom), 8.15 s (1H, NH, imidazole), 9.12 s (1H, NH, indole). Mass spectrum: m/z 425 [M]+.
Biological evaluations. The antimicrobial and antioxidant activities of compounds 3–7 in vitro were assayed by the cup-plate method as reported in [10, 11]. The detailed procedures are given in Supplementary Materials.
CONCLUSIONS
A rapid, convenient, and environmentally friendly microwave-assisted procedure has been developed for the synthesis of new biologically active thiazolidin-4-one, triazole, azitidine-2-one, and thioether derivatives bearing indole and benzimidazole scaffolds. Five of the twenty synthesized compounds exhibited considerable antimicrobial activity comparable to the activity of reference drugs, and some of the compounds showed excellent antioxidant activity. Thus, the synthesized compounds may be promising candidates for new antimicrobial drugs and further investigations in the field of medicinal chemistry.
REFERENCES
Rathod, A.S., Reddy, P.V., and Biradar, J.S., Russ. J. Org. Chem., 2020, vol. 56, p. 662. https://doi.org/10.1134/S1070428020040156
Rathod, A.S., Godipurge, S.S., and Biradar, J.S., Russ. J. Gen. Chem., 2018, vol. 88, p. 1238. https://doi.org/10.1134/S1070363218060324
Rathod, A.S. and Biradar, J.S., Russ. J. Gen. Chem., 2018, vol. 88, no. 10, p. 2190. https://doi.org/10.1134/S1070363218100262
Rathod, A.S. and Biradar, J.S., Russ. J. Gen. Chem., 2020, vol. 90, no. 1, p. 135. https://doi.org/10.1134/S1070363220010211
O’Neil, M.J. and Smith, M., The Merck Index, 2001, 13th ed., P-1785, 10074.
Amari, M., Fodili, M., Nedjar-Kolli, B., Hoffmann, A.P., and Périé, J., J. Heterocycl. Chem., 2002, vol. 39, no. 4, p. 811. https://doi.org/10.1002/jhet.5570390429
Köhler, P., Int. J. Parasitol., 2001, vol. 31, no. 4, p. 336. https://doi.org/10.1016/s0020-7519(01)00131-x
Mavrova, A.T., Anichina, K.K., Vuchev, D.I., Tsenov, J.A., Denkova, P.S., Kondeva, M.S., and Micheva, M.K., Eur. J. Med. Chem., 2006. vol. 41, no. 12, p. 1412. https://doi.org/10.1016/j.ejmech.2006.07.005
Campbell, W.C. and Denham, D.A., Trichinella and Trichinosis, Campbell, W.C., Ed., New York: Plenum, 1983, p. 340.
Rathod, A.S., Godipurge, S.S., and Biradar, J.S., Int. J. Pharm. Pharm. Sci., 2017, vol. 9, no. 12, p. 233. https://doi.org/10.22159/ijpps.2017v9i12.21970
Rathod, A.S., Godipurge, S.S., and Biradar, J.S., Asian J. Pharm. Pharmacol., 2017, vol. 3, no. 6, p. 229.
Biradar, J.S. and Sharanbasappa, B., Green Chem. Lett. Rev., 2009, vol. 2, no. 4, p. 237. https://doi.org/10.1080/17518250903393890
Biradar, J.S. and Sharanbasappa, B., Synth Commun., 2011, vol. 41, p. 885. https://doi.org/10.1080/00397911003707071
Biradar, J.S. and Sasidhar, S., Eur. J. Med. Chem., 2011, vol. 46, p. 6112. https://doi.org/10.1016/j.ejmech.2011.10.004
Sasidhar, S. and Biradar, J.S., Med. Chem. Res., 2013, vol. 22, p. 3518. https://doi.org/10.1007/s00044-012-0370-x
Rathod, A.S., Ph.D. Thesis, Gulbarga University, 2019.
Bhrighu, B., Siddiqui, N., Pathak, D., Alam, M.S., Ali, R., and Azad, B., Acta. Pol. Pharm., 2012, vol. 69, p. 53.
Demirayak, S., Karaburun, A.C., Kayagil, I., Erol, K., and Sirmagul, B., Arch. Pharmacal Res., 2004, vol. 27, no. 1, p. 13. https://doi.org/10.1007/BF02980038
Saundane, A.R. and Mathada, K.N., Monatsh. Chem., 2015, vol. 146, p. 1751. https://doi.org/10.1007/s00706-015-1440-9
Hiremath, S.P., Badami, P.S., and Purohit, M.G., Indian J. Chem., Sect. B, 1985, vol. 24, p. 1235.
ACKNOWLEDGMENTS
The authors thank the Chairman, Department of Chemistry, Gulbarga University, Kalaburagi, for providing laboratory facilities, Chairman, Department of Microbiology and Biotechnology, Gulbarga University, Kalaburagi, for providing laboratory facilities to carry out antimicrobial assays, and Director, SAIF, Punjab (India), and director IICT, Hyderabad, for obtaining spectral data.
Funding
A.S. Rathod thanks the UGC-BSR (SRF), no. F.25-1/2013-14 (BSR)/no. F 7-226/2009, dated Nov 19, 2014, New Delhi (India) for financial support,
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest
Supplementary information
Rights and permissions
About this article
Cite this article
Rathod, A.S., Biradar, J.S. Green Approach to the Synthesis of New Indole and Benzimidazole Analogs and Their Biological Evaluation. Russ J Org Chem 57, 1540–1551 (2021). https://doi.org/10.1134/S1070428021090220
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021090220