Abstract
A method for production of polyurethane coatings with low surface energy modified with a block copolymer polydimethylsiloxane–polyphenylsilsesquioxane is presented. Surface characteristics of coatings were studied by methods of measuring the contact angle, attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy and scanning electron microscopy with X-ray spectral microanalysis, and the values of static contact angles and surface energy were obtained. The effect of pigmentation on wetting and physicomechanical characteristics of low-energy coatings has been investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Hydrophobic coatings with low surface energy are used in various industries: textile, automotive, shipbuilding, aerospace, as well as in optics, microelectronics, and many others [1]. Polyurethane materials are characterized by high adhesion to various substrates, strength, flexibility, and high resistance to various aggressive factors, however, polyurethane coatings do not have hydrophobic properties and the contact angle rarely reaches values higher than 90°. Application of modifiers that reduce surface energy and increase hydrophobicity and also fillers improves the performance of polyurethane coatings. Rabea et al. [2] introduced silicone-modified polyacrylate and silicon dioxide into a polyurethane matrix, as a result, they obtained hydrophobic materials with a contact angle of 104° and increased physical and mechanical characteristics. The hydrophobic properties of the polyurethane matrix were enhanced by introducing polydimethylsiloxane and a mixture of silicon dioxide and silver [3]. Modification of the polyurethane matrix with polydimethylsiloxane and a mixture of silicon dioxide and aluminum leads to an increase in the value of the contact angle and roll-off angle up to 159° and 4°, respectively [4]. The introduction of polyhedral oligomeric silsesquioxane into the polyurethane composition improves physical and mechanical characteristics, reduces water absorption and increases the hydrophobicity of coatings [5].
This work is aimed at investigation of the possibility of creating hydrophobic polyurethane coatings based on a hydroxyl-containing acrylic copolymer and a polyisocyanate hardener by modifying a block copolymer polydimethylsiloxane–polyphenylsilsesquioxane with small additives.
EXPERIMENTAL
As objects of study we used hydroxyl-containing acrylic copolymer Eterac 7333-x 60 (50 wt % solution in o-xylene), the content of –OH-group 2.7% (Eternal Materials); aliphatic polyisocyanate hardener hexamethylene diisocyanate biuret Desmodur N75 (75 wt % solution in o-xylene), content of –NCO-groups 16.5% (Bayer); organosilicon block copolymer polydimethylsiloxane–polyphenylsilsesquioxane SilPol, Mn = 38.5 ×103, Mw = 105.6 × 103, the ratio of the lengths of the sequences of flexible polydimethylsiloxane and rigid polyphenylsilsesquioxane blocks 61 and 25, respectively (FSUE NIISK named after S.V. Lebedev); catalyst for urethane formation dibutyltin dilaurate (0.1 wt % solution in o-xylene) TIB KAT 218 (TIB Chemicals); TiO2 Tiona 595, average particle size 0.26 μm (Cristal); talc Finntalc M05N of average particle size 2 μm (Finntalc) and n-hexadecane (chemically pure) (JSC “Ecos-1”). The listed materials were used without additional purification and processing.
Compositions for production of films and coatings were prepared according the following procedure: SilPol was added to the Eterac 7333-x-60 solution in an amount of 1 to 15 wt % with mechanical blending until an opalescent solution was obtained. A Desmodur N75 solution at an –OH : –NCO = 1 : 1.1 ratio and a TIB KAT 218 solution were added to the prepared blend. Then the composition was additionally subjected to mechanical blending for 5 min.
Pigmented compositions were prepared by adding powdered Finntalc M05N and Tiona 595 to the Eterac 7333-x-60 solution in an amount of 5 and 27 wt %, respectively, and dispersed in a bead mill for 30 min. The size of the largest agglomerates after dispersing the mixture was 25 ± 2.5 μm. It was determined with a Klin grindometer (Constanta LLC). A block copolymer of SilPol, Desmodur N75, and TIB KAT 218 was introduced into the composition as described above.
The resulting compositions were applied to the surface of steel or glass plates prepared in accordance with GOST (State Standard) 9.402-2004 “ESZKS. Paint and varnish coatings. Preparation of metal surfaces for painting” and GOST 8832–76 “Methods of obtaining paint and varnish coatings for testing,” using the KA1-21 applicator (LLC RNPO RusPribor) with a gap thickness of 200 μm for the film. Films were produced by casting the compositions onto aluminum foil prepared in accordance with GOST 9.402-2004, followed by peeling.
In all cases, the curing of the coatings was carried out under normal conditions for 8 days.
The thickness of the cured coatings on a metal substrate was determined by a Mega Check FN thickness gauge (List-Magnetik GmbH). The thickness of the obtained coatings was 60 ± 15 μm. The thickness of the cured peeled off films and coatings on a glass substrate was determined by a Digital Micrometer Schut 908.750 (Schut Geometrical Metrology). The thickness of the obtained peeled off films was 180 ± 27 μm.
The static contact angles were measured with a KRÜSS DSA25 device (KRÜSS GmbH) with Advance software. The static contact angles of water and n-hexadecane were determined by the sessile drop method (the drop volume was 1 μL). The determination was carried out at 5–10 different points on the surface of the coating under study, afterwards the average value was found. The error was no more than 2°. The surface energy of the coatings was calculated by the Owens–Wendt–Rabel–Kaellble (OWRK) method.
The Fourier transform infrared (FTIR) spectra of the films were recorded on an IRTracer-100 spectrometer (Shimadzu Europa GmbH) with a Quest (Specac) attachment for the attenuated total internal reflection (ATR). The crystal material was diamond. The angle of incidence of the IR beam on the crystal was 45°. Penetration depth was 2 μm.
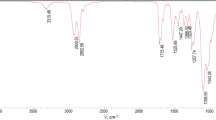
IR spectrum of the cured polyurethane film (cm–1): 3379 (νNH), 3086–3027 (νCH Ar ring), 2934–2864 (ν –CH2–, –CH3), 2272 (ν –NCO), 1729–1699 [ νCO in –NH–C(O)O–], 1641 [νCO in –C(O)O–], 1521 [νN–H in –NH–C(O)O–], 1454 (ν –CH2–) , 1380 (δ –CH3), 1340 (–NCO), 1249–1167 (νC–O in –C–O–C–), 1122 (νCH Ar ring), 1076–1028 [νCO in –C(O)О–R], 762–702 (νCH Ar ring).
IR spectrum of organosilicon block copolymer (cm–1): 3737 [–OH in (SiO3)2], 3078–3058 (νCH Ar ring), 2965–2912 (νCH3), 1433–1413 [δ –CH3 in –Si (CH3)], 1261 [δ –CH3 in –Si(CH3)], 1133 (νCH Ar ring), 1101–1016 (νSi–O–Si), 870 [ν –CH3 in –Si(CH3)2], 803 [νSi–C in –Si(CH3)2)], 738 (νCH Ar ring), 696 (νSi–C), 507 (νSi–O–Si).
The compatibility of the SilPol additive with the polyurethane matrix was determined with a KFK-2 photocolorimeter (LLC Zapadpribor) by the change in the optical density of the cured films on glass plates of 1.25 mm thickness.
Optical micrographs were obtained by a PMT-3 device (JSC LOMO) and an Altami USB 3150R6 ½ CMOS digital camera (LLC Altami ). Image processing was performed with Altami Studio and ImageJ software.
Images of the film surface were taken by SEM on a TESCAN VEGA 3 SBH (TESCAN) scanning electron microscope at an accelerating voltage of 20 kV, a focal length of 15 mm, and a probe current of 17 pA. The samples were fixed on double-sided tape, after which a carbon coating of 5–20 nm thickness was deposited onto the surface using a Q150RE (Quorum) setup. The image was recorded by a backscattered electron detector. Elemental analysis of the samples was carried out by X-ray spectral microanalysis (X-ray microanalysis) using an AdvancedAztecEnergy (Oxford Instruments) energy dispersive spectrometer attachment.
The physicomechanical properties of the coatings were characterized according to the common test methods for paints and varnishes in accordance with GOST 4765-73 “Method for determining the impact strength,” GOST 31149-2014 “Determination of adhesion by the cross-cut method,” and GOST R 52740-2007 “Method for determining the strength of a coating bending around a cylindrical rod.”
RESULTS AND DISCUSSION
The modification of compositions with low surface energy compounds is one of the methods for production of hydrophobic coatings. In the work an organosilicon block copolymer SilPol was used as a modifier. The values of the water contact angle, dispersion (γD) and polar (γP) components of the surface energy for coatings prepared from a 20 wt % SilPol solution were 111°, 19.1, and 0.2 mJ m–2, respectively.
For the hydrophobization of polyurethane coatings, the initial compositions were supplemented with an organosilicon polymer SilPol in amounts ensuring its content in the cured coating from 1 to 15 wt %. With a modifier content of 1 wt %, the static contact angle increased from 88° to 102°. At 2 wt %, the curve reached a plateau of hydrophobicity with 106° and remained unchanged with a further increase in the SilPol content (Fig. 1).
Also, at 1 wt % SilPol, the surface energy decreased by 13% (Fig. 2). The main contribution to the decrease in the surface energy is made by its polar component, which decreases by 77.5% from 4.0 to 0.9 mJ m–2, while the dispersion component insignificantly changes from 23.0 to 22.6 mJ m–2. A further increase in the SilPol content above 2 wt % does not lead to a significant change in the surface energy and its components.
The data presented allow conclusion that the introduction of 2–3 wt % SilPol is sufficient to achieve maximum hydrophobicity, then an increase in its content has no significant effect due to saturation of the coating surface with an organosilicon block copolymer, which is consistent with the data of [7, 8].
Optical micrographs of cured films with different SilPol content indicate a significant change in their morphology depending on the modifier content (Fig. 3). The unmodified polyurethane film is homogeneous and does not contain any inclusions (Fig. 3a). With the introduction of 1.5 wt % SilPol, a multitude of spherical inclusions appear (Fig. 3b), which increase in size and quantity with a rise in the modifier content to 3 and 7 wt % (Figs. 3c, 3d).
The chemical composition of the film surface at the interface with air was additionally investigated by the SEM-X-ray spectral microanalysis; (Fig. 4; Tables 1, 2). As the amount of SilPol increases from 1.5 to 7 wt %, the average silicon content in the surface layer of the film increases from 0.34 to 0.88 wt % (Table 1). When analyzing the surface of the modified film at individual points (Figs. 4a, 4b), the silicon content varies from 0.35 to 21.77 wt % (Table 2). Significant differences in the elemental composition at different points of the surface confirm that during the formation of the polyurethane film, a microphase separation occurs between the polyurethane matrix and the organosilicon block copolymer. This fact may be due to incompatibility of the components, which leads to the formation of areas with an increased content of the modifier and a change in the surface structure of the polyurethane coating due to a rise in the SilPol content in the surface layer. It should be noted that the calculated silicon content in the SilPol block copolymer is ~28.3 wt %. With its introduction into the coating in an amount of 1.5, 3.0, or 7.0 wt % and the assumption of a uniform distribution in the film volume, the calculated silicon content would be ~0.42, ~0.85, and ~1.98 wt %, respectively. The experimentally determined silicon content is 0.34, 0.63, and 0.88 wt %.
The compatibility of the polyurethane matrix and SilPol was determined by the change in the optical density of the films. The results obtained (Fig. 5) evidence a significant increase in the optical density of the cured films up to 0.65 units with an increase in the content of the block copolymer to 7.5 wt % and additionally confirm the heterogeneity of their structure due to the incompatibility of the polyurethane matrix and the modifier.
Moreover, we studied the wetting of peeled off films obtained on an aluminum substrate at the coating–air and coating–substrate interfaces (Fig. 6). The 8° difference between the contact angle of the unmodified coating at the coating–air (87°) and coating–substrate (79°) interface can be explained by the various distribution patterns of polyurethane segments at the interfaces, as well as a partial change in the surface composition when the film peels off from aluminum substrate [9, 10].
With an increase in the content of the organosilicon polymer to 2 wt %, the contact angle of water at the coating–air interface increases to 106° and only to 97° at the coating–substrate interface. The introduction of more than 2 wt % SilPol does not affect the hydrophobicity of the coating at the coating–air interface, while the contact angle continues to increase at the coating–substrate interface. Saturation of the lower layers of the SilPol coating occurs only when the modifier content is 10 wt %. It can be assumed that when the modifier content is less than 2 wt %, not only preferential migration to the interface with air proceeds, but also partial wetting of the substrate surface [11]. Consequently, the saturation of both interfaces is not the same and depends on the content of the organosilicon block copolymer, which substantiates the complex nature of the additive distribution. Thus, the totality of the results obtained indicates the formation of a heterophase structure of the films; however, complete self-stratifying of the system does not occur.
To further study the nature of the distribution of the organosilicon block copolymer, the surface layers of the films were examined by FTIR spectroscopy.
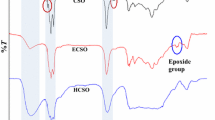
The analysis of the structure of the surface layers of the modified polyurethane film can be carried out in the range of absorption bands 1262, 1135, and 805–802 cm–1, which are characteristic of –Si–(C6H5) and –Si–(CH3) bond vibrations (Fig. 7a). In the FTIR spectra of polyurethane films with 5 wt % SilPol (Fig. 7b), the intensity of these absorption bands at the coating–substrate interface is lower than at the coating–air interface, which evidences a higher content of organosilicon polymer at the coating–air interface. With increasing the SilPol content to 15 wt %, the intensity of absorption bands for the coating–air interface varies insignificantly. At the same time, the intensity of absorption bands for the coating–substrate interface approaches those at the coating–air interface due to saturation of the lower layers of the film. Thus, depending on the SilPol content in the composition, the presence of the organosilicon block copolymer at the interfaces is different, which agrees with the results obtained in the film surface examination by the contact angle method.
One of the disadvantages of many hydrophobic coatings is their low strength and adhesion to the substrate [12, 13]. With increasing the SilPol content the flexural strength of the coatings remains high, but the adhesion and impact strength deteriorate significantly (Table 3), which can be explained by the accumulation of the modifier at the coating–substrate interface.
The introduction of a mixture of TiO2 (27 wt %) and talc (5 wt %) does not affect the wetting degree of the coatings (Table 4), while adhesion and impact strength rise (Table 5).
With increasing the content of the organosilicon block copolymer in the pigmented coating, the elastic modulus, which can be indirectly characterized by flexural strength (Table 5), increases as compared to varnish coatings (Table 3). Strengthening and an increase in the modulus of samples in pigmented coatings can be associated with the additional formation of adhesive bonds between the polymer binder and the filler [14, 15], as well as a change in the morphology of the coatings due to the presence of a heterophase structure.
CONCLUSIONS
The introduction of an organosilicon block copolymer of up to 2–3 wt % into a composition based on a hydroxyl-containing acrylic copolymer and an aliphatic polyisocyanate makes it possible to produce coatings with low surface energy. In lacquer films, a microheterophase structure is formed due to the incompatibility of the block copolymer with the polyurethane matrix. The enrichment of the near-surface layers of the coating at the interfaces with both air and, to a lesser extent, with the substrate results in a decrease in the adhesion and impact strength of the coatings. The introduction of a pigment mixture of TiO2 and talc into the modified compositions afforded coatings with optimal properties.
REFERENCES
Ganesh, V.A., Raut, H.K., Nair, A.S., and Ramakrishna, S., J. Mater. Chem., 2011, vol. 21, no. 41, pp. 16304–16322. https://doi.org/10.1039/C1JM12523K
Rabea, A.M., Mohseni, M., Mirabedini, S.M., and Tabatabaei, M.H. , Appl. Surf. Sci., 2012, vol. 258, no. 10, pp. 4391–4396. https://doi.org/10.1016/j.apsusc.2011.12.123
Lv, D., Fang, N. and Zhang, W., Infrared Phys. Technol., 2020, vol. 108, p. 103351. https://doi.org/10.1016/j.infrared.2020.103351
Zhang, W., Jiang, S., and Lv, D., Prog. Org. Coat., 2020, vol. 143, p. 105622. https://doi.org/10.1016/j.porgcoat.2020.105622
Rahman, M.M., Zahir, M.H., Haq, M.B., Madhan Kumar, A., Arafat, M.E., and Rabbani, M.M., Int. J. Polym. Anal. Charact., 2020, vol. 25, no. 5, pp. 385–395. https://doi.org/10.1080/1023666X.2020.1796106
Voznyakovskii, A.P., Kudoyarova, V.K., Kudoyarov, M.F., and Patrova, M.Y., Phys. Solid State, 2017, vol. 59, no. 8, pp. 1656–1661. https://doi.org/10.1134/S1063783417080327
Ramezanzadeh, B., Mohseni, M., Rabea, A.M., and Yarih H., Int. J. Adhes. Adhes., 2011, vol. 31, no. 7, pp. 775–783. https://doi.org/10.1016/j.ijadhadh.2011.07.007
Yilgör, E. and Yilgör, I., Prog. Polym. Sci., 2014, vol. 39, no. 6, pp. 1165–1195. https://doi.org/10.1016/j.progpolymsci.2013.11.003
Chattopadhyay, D.K. and Raju, K.V.S.N., Prog. Polym. Sci., 2007, vol. 32, no. 3. pp. 352–418. https://doi.org/10.1016/j.progpolymsci.2006.05.003
Mashlyakovskii, L.N., Koz’mina, N.S., Egorova, N.A., and Khomko, E.V., Russ. J. Appl. Chem., 2018, vol. 91, no. 4, pp. 629–640. https://doi.org/10.1134/S1070427218040158
Beaugendre, A., Degoutin, S., Bellayer, S., Pierlot, C., Duquesne, S., Casetta, M., and Jimenez, M., Prog. Org. Coat., 2017, vol. 110, pp. 210–241. https://doi.org/10.1016/j.porgcoat.2017.03.011
Verho, T., Bower, C., Andrew, P., Franssila, S., Ikkala, O., and Ras, R.H., Adv. Mater., 2011, vol. 23, no. 5, pp. 673–678. https://doi.org/10.1002/adma.201003129
Ellinas, K., Tserepi, A., and Gogolides, E., Adv. Colloid Interface Sci., 2017, vol. 250, pp. 132–157. https://doi.org/10.1016/j.cis.2017.09.003
Malik, M. and Kaur, R., Adv. Polym. Technol., 2018, vol. 37, no. 1, pp. 24–30. https://doi.org/10.1002/adv.21637
Vaimakis-Tsogkas, D.T., Bekas, D.G., Giannakopoulou, T., Todorova, N., Paipetis, A.S., and Barkoula, N.M. , Mater. Chem. Phys., 2019, vol. 223, pp. 366–373.
ACKNOWLEDGMENTS
We express our gratitude to FSUE NIISK named after S.V. Lebedev for the product provided and information on the parameters of the silicone polymer SilPol, as well as to the engineering center of St. Petersburg State Technological Institute (Technical University).
Funding
The work was carried out within the framework of a comprehensive project for the creation of high-tech production of the Ministry of Education and Science of the Russian Federation (contract no. 03.G25.31.0237) and the state assignment of the Ministry of Education and Science of the Russian Federation (project no. 11.5362.2017/8.9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest requiring disclosure in this article.
Additional information
Translated from Zhurnal Prikladnoi Khimii, No. 5, pp. 646–654, January, 2021 https://doi.org/10.31857/S0044461821050133
Rights and permissions
About this article
Cite this article
Erofeev, D.A., Mashlyakovskii, L.N., Khomko, E.V. et al. Low Surface Energy Polyurethane Coatings Based on Acrylic Copolymer and Polyisocyanate Modified with an Organosilicon Block Copolymer. Russ J Appl Chem 94, 647–655 (2021). https://doi.org/10.1134/S107042722105013X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042722105013X