Abstract
The structure of an active diluent influences the rheological properties of epoxy compounds. The use of a linear alkyl glycidyl ether allows preparation of compounds with the lowest dynamic viscosity. As the active diluent content is increased from 0 to 20%, the period in which the network polymer is formed in the course of curing of epoxy compounds increases from 5 to 30 min because of a decrease in the rate of consumption of epoxy groups in the compound with an increase in the active diluent content. The elastic-strain and thermal properties of epoxy polymer films were studied. When using a linear alkyl glycidyl ether as an active diluent, the ultimate strength and glass transition temperature as functions of the diluent content pass through a minimum. Introduction of monofunctional active diluents into the compound leads to a decrease in the elastic modulus. An increase in their content in the compound from 5 to 20% does not affect the elastic modulus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Much attention is paid today to the development of paint-and-varnish materials with low content of volatile organic substances. This problem can be solved by using aqueous dispersions of polymers or preparing organic-soluble systems containing no classical organic solvents that are evaporated into air in the course of curing. The viscosity of such systems can be controlled using so-called active diluents, which become incorporated in the formed network structure upon curing. Widely used systems of this type include unsaturated polyesters (active diluents in such systems are unsaturated monomers, e.g., styrene, methyl methacrylate, etc.) [1–3], acrylic oligomers (with polyols derived from ε-caprolactone as active diluents) [4–6], and epoxy oligomers (possible active diluents in this case are, e.g., aliphatic epoxy oligomers or monofunctional compounds containing epoxy groups) [7–9]. However, in the above papers, an active diluent is considered as an alternative to commonly used solvents only from the viewpoint of controlling the viscosity of the material. The effect of the structure and functionality of the active diluent on the properties of the coatings formed has not been evaluated, which does not allow correct evaluation of changes in the properties of coatings formed from the given compound. On the other hand, incorporation of an active diluent into a polymer network can influence its structure and hence its operation characteristics.
This study deals with the effect of active diluents on the properties of epoxy compounds and polymer films based on them.
EXPERIMENTAL
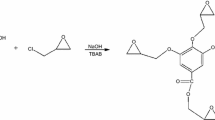
As investigation objects we used low-molecular-mass epoxy oligomers based on 4,4′-isopropylidenediphenol with the epoxy number of 23% (epoxy equivalent 187 g-equiv–1) (EO-1) (DER-331, Dow Chemical) and on 4,4′-methylenediphenol with the epoxy number of 25.2% (170.5 g-equiv–1) (EO-2) (DER-354, Dow Chemical). As active diluents we used alkyl glycidyl ether with the С12–С14 alkyl fragment (AD-1) (epoxy equivalent 320 g-equiv–1) (Eposir 7106, SIR INDUSTRIALE), propylene glycol diglycidyl ether (AD-2) (epoxy equivalent 320 g-equiv–1) (DER-732P, Dow Chemical), and glycidyl neodecanoate (AD-3) (epoxy equivalent 239 g-equiv–1) (Cardura E10P, Momentive Speciality Chemicals). The content of the active diluent in the epoxy compound was varied from 0 to 20%. As curing agents we used cycloaliphatic diamines based on isophoronediamine, containing benzyl alcohol: АСА-1 (CeTePox 1312 FS H, Aditya Birla Group Company), АСА-2 (CeTePox 1588 L H, Aditya Birla Group Company), and АСА-3 (ITAMINE CA54, DDChem), with the H-equivalents of 95, 113, and 93 g-equiv–1, respectively. The content of the amine curing agent was calculated from the epoxy and H-equivalents of the oligomer and curing agent, taking into account the amount of the active diluent added. Curing of the epoxy compounds was performed at 60 ± 2°С. Epoxy compounds were prepared by mixing an epoxy oligomer with the required amount of an active diluent, after which the chosen curing agent was added in the stoichiometric amount. To obtain free films, epoxy compounds were applied onto a polyethylene support using a screw applicator with a gap of 100 μm. The films were cured for 4 h.
The rheological properties of epoxy compounds were studied by rotary viscometry on a Rheomat RM180 device with a system of coaxial cylinders (measurements were performed 10 min after compounding). The content of epoxy groups in a compound in the course of its curing was determined by nonaqueous potentiometric titration with a perchloric acid solution (analytically pure grade, ZAO Kupavnareaktiv) [10]. The rate of consumption of epoxy groups was determined from the dependence of the content of epoxy groups on the curing time. The content of the network polymer in an epoxy compound was determined by gel–sol analysis in a continuously operating Soxhlet extractor. The solvent for the extraction was acetone (analytically pure grade, AO EKOS-1); the extraction was performed for 6 h [11]. The glass transition temperatures of the formed epoxy materials were determined by differential scanning calorimetry with a DSC 822e device (Mettler Toledo). Tests were performed in the temperature interval 0–150°С under nitrogen. The scanning rate was 10 deg min–1. The elastic-strain characteristics of free epoxy films were evaluated by uniaxial extension with a ZWICK/ROEL TC-FR010 universal testing machine. The extension velocity was 1 mm min–1.
RESULTS AND DISCUSSION
Introduction of an active diluent into an epoxy oligomer should alter the rheological properties of the epoxy oligomer. The structure of the active diluent should determine changes in the rheological characteristics, influencing the process of the material application. Indeed, introduction of an active diluent into an epoxy oligomer decreases the viscosity of the compound, and variation of the active diluent structure leads to variation of the rheological characteristics. In particular, introduction of 10% (here and hereinafter, wt %) AD-1 into the compound decreases its viscosity by a factor of 4 (Fig. 1a, curve 1).
Introduction of bifunctional AD-2 also decreases the viscosity of the system (Fig. 1a, curve 2). However, to reach the same viscosity of the compound, AD-2 should be added in approximately 3 times larger amount than AD-1.
This can be attributed to higher viscosity (65 mPa s) of AD-2 compared to AD-1 (11 mPa s). Deviation of the viscosity of the compounds from the additivity suggests changes in the intermolecular interactions in the compound. In the condensed state, molecules of organic compounds are bound to each other by a system of intermolecular interactions to form associates whose topology can be very different depending on the number (n) of intermolecular interaction sites, their energy, and other factors [12, 13]. Active diluents AD-1 and AD-2 contain long hydrocarbon fragments capable of only weak (dispersion) intermolecular interactions. Their introduction into epoxy oligomers decreases the total level of intermolecular interactions and, as a consequence, can also decrease the viscosity of the compounds. More significant decrease in the viscosity on introducing AD-1 is associated with the presence of only one site of strong intermolecular interaction (epoxy group) in the molecule, whereas in the molecule of AD-2 there are two such sites.
Introduction of a monofunctional branched diluent, AD-3, leads to a decrease in the viscosity of the compound to the extent comparable to that ensured by the linear active diluent AD-1 (Fig. 1a, curve 3). AD-3 active diluent is somewhat inferior to AD-1 in the dilution efficiency, probably because of smaller size of the hydrocarbon fragment and compactness of the molecule. It should be noted that introduction of various active diluents decreases the viscosity throughout the examined composition range. However, irrespective of the structure of the monofunctional active diluent, the most pronounced decrease in the viscosity on its introduction is observed at its content in the compound of up to 10%.
Similar relationships are observed for epoxy compounds containing 4,4′-methylenediphenol diglycidyl ether (Fig. 1b). However, because of lower initial viscosity of this oligomer (by a factor of approximately 2.5), active diluents do not demonstrate such performance as in the compounds based on 4,4′-isopropylidenediphenol (Fig. 1a). The strongest changes in the rheological properties are observed when using monofunctional active diluents AD-1 and AD-3, which is due to their lower viscosity (11 and 7 mPa s, respectively) compared to the bifunctional diluent AD-2 (65 mPa s). It should be taken into account that introduction of an active diluent containing reactive epoxy groups should affect the curing of the compounds.
Further increase in the active diluent content of the compound is not appropriate for several reasons. First, a decrease in the viscosity with an increase in the active diluent content to 25% and higher values influences the compound viscosity insignificantly compared to the initial values (the dynamic viscosity of EO-1 without dilution and with 10, 20, and 30% AD-1 is 8.66, 1.64, 0.51, and 0.23 Pa s, respectively). Similar trend is observed with the other active diluents. Second, high content of active diluents (>20%) can lead to significant deceleration of curing and deterioration of operation characteristics. Third, the production cost of the compound significantly increases.
The curing agent used influences the curing of the epoxy compounds (Fig. 2). With ACA-1, the compound curing rate and the network polymer content are lower than with АСА-2 and АСА-3. This trend is observed for the compounds based both on 4,4′-isopropylidenediphenol (Fig. 2a) and on 4,4′-methylenediphenol (Fig. 2b).
Viscosity of the epoxy oligomer based on (a) 4,4′-isopropylidenediphenol and (b) methylenediphenol as a function of active diluent content. 25 ± 1°С, shear rate γ = 100 s–1; the straight lines correspond to the additive plots. (1) Alkyl glycidyl ether with С12–С14 alkyl fragment, (2) propylene glycol diglycidyl ether, and (3) glycidyl neodecanoate.
It should be noted that, in curing of compounds based on EO-1, the limiting gel fraction content of the compounds containing АСА-2 and АСА-3 is the same. In going to EO-2, the gel fraction content of the compound with АСА-3 becomes higher than that of the compound with АСА-2. This difference is due to the fact that EO-2 is considerably less viscous than EO-1; hence, there will be weaker diffusion hindrance at high conversions, taking into account the fact that the dynamic viscosity of АСА-3 (6.8 Pa s) is higher than that of АСА-2 by an order of magnitude (0.36 Pa s). The steric hindrance to curing of the compound with АСА-3 is confirmed by the data in Fig. 3. The highest initial reaction rate in the case of both EO-1 and EO-2 is observed when using АСА-3. However, the network polymer content when using АСА-3 in the compound with EO-1 remains the same as when using АСА-2, which is caused by diffusion-topological limitations of the curing reaction. The use of the less viscous EO-2 partially lifts these limitations, which leads to an increase in the gel fraction content of the compounds (Fig. 2b).
The curing should be influenced both by the active diluent amount and by its functionality. Monofunctional diluents can terminate propagating chains, which can lead to changes both in the topology of the three-dimensional network and in the network polymer content as a whole. The effect of active diluents on the properties of epoxy compounds was studied using АСА-1, because this curing agent has the simplest composition, containing only a single amine-containing product, isophoronediamine.
An increase in the content of monofunctional АР-1 leads to a decrease in the gel fraction content of the coatings (Fig. 4a). This is caused by the fact that the monofunctional epoxy oligomer, when reacting with a curing agent, will yield a product that is washed out with acetone in the course of the extraction. The probability of the formation of such products increases with an increase in the active diluent content of the compound. It should also be noted that introduction of the monofunctional oligomer leads to an increase in the time of the network polymer formation. This is caused by an increase in the content of epoxy groups reacting with the curing agent without formation of the network structure.
Gel fraction content as a function of curing time for compounds containing the epoxy oligomer based on 4,4′-isopropylidenediphenol, CeTePox 1312 FS H, and (a) alkyl glycidyl ether with С12–С14 alkyl fragment or (b) propylene glycol diglycidyl ether. Active diiluent content, %: (1) 0, (2) 5, (3) 10, (4) 15, and (5) 20.
It is known [14] that aliphatic and monoepoxy compounds exhibit lower reactivity. Presumably, their introduction into the system will affect the rate of consumption of the epoxy groups in the reaction with the amine curing agent. Indeed, the initial rate of the consumption of epoxy groups decreases with an increase in the active diluent content, with the bifunctional AD-2 decreasing this parameter to a greater extent compared to AD-1 (Fig. 5).
It should be noted that the use of the bifunctional active diluent AD-2 also leads to a decrease in the network polymer content (especially at AD-2 content higher than 10%) relative to the initial compound (Fig. 4b). This is caused by the fact that AD-2 under the curing conditions used in our experiments forms the network polymer very slowly (after 8 h, the content of the network polymer in the AD-2 + АСА-1 compound, determined by gel–sol analysis, does not exceed 5%). With the epoxy oligomer based on 4,4′-methylenediphenol, the dependences of the network polymer content on the active diluent content of the system were similar.
Changes in the curing process, caused by introduction of an active diluent and by structural features of cross-linking agents, should influence the properties of the cured epoxy materials. For all the samples, introduction of an active diluent leads to a decrease in the elastic modulus of the polymer films (Figs. 6a, 6b, 6e, 6f). An increase in the content of AD-1 from 5 to 20% in the compound based on EO-1 does not lead to a decrease in the elastic modulus of the cured materials (Fig. 6a, curve 1). The same pattern is observed when using AD-1 and AD-3 in the compounds based on EO-2 (Fig. 6e, curves 1 and 3). Furthermore, for the film formed from the compound based on EO-2, AD-3, and АСА-3, the elastic modulus is independent of the active diluent content (Fig. 6f, curve 3). This fact is unusual, because an increase in the content of the component acting as a chain-terminating agent should lead to a decrease in the thickness of cross-linking of epoxy polymer materials or to physical plasticization of the films obtained. An increase in the active diluent content also leads to a decrease in the ultimate strength of epoxy polymer materials (Figs. 6c, 6d, 6g, 6h) relative to the compounds without active diluent. A decrease in the elastic modulus is due to the fact that the forming polymer has lower thickness of the chemical network; hence, the coating will be more elastic. With AD-1, the ultimate strength as a function of the active diluent content passes through a minimum: Introduction of up to 10% AD-1 into the compound leads to a decrease in the ultimate strength, probably due to a decrease in the chemical network thickness, whereas introduction of larger amounts of AD-1 leads to an increase in the ultimate strength of the film, probably due to the antiplasticization effect (Figs. 6c, 6g, curve 1).
Influence of the active diluent content on the (a, b, e, f) elastic modulus and (c, d, g, h) ultimate strength of epoxy polymer films. (a, c) Films based on 4,4′-isopropylidenediphenol diglycidyl ether, CeTePox 1312 FS H, and various active diluents: (1) alkyl glycidyl ether with С12–С14 alkyl fragment, (2) propylene glycol diglycidyl ether, and (3) glycidyl neodecanoate; (b, d) dilms based on on 4,4′-isopropylidenediphenol diglycidyl ether, glycidyl neodecanoate, and various curing agents: (1) CeTePox 1312 FS H, (2) CeTePox 1588 L H, and (3) ITAMINE CA54; (e, g) films based on 4,4′-methylenediphenol diglycidyl ether, CeTePox 1312 FS H, and varius active diluents: (1) alkyl glycidyl ether with С12–С14 alkyl fragment, (2) propylene glycol diglycidyl ether, and (3) glycidyl neodecanoate; (f, h) dilms based on on 4,4′-methylenediphenol diglycidyl ether, glycidyl neodecanoate, and various curing agents: (1) CeTePox 1312 FS H, (2) CeTePox 1588 L H, and (3) ITAMINE CA54.
This trend is observed both with EO-1 and with EO-2. At the diluent content of the compound lower than 10%, which is the most acceptable level from the viewpoint of rheological studies, the least pronounced decrease in the elastic-strain properties is observed with AD-3 (Fig. 6).
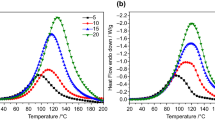
A decrease in the elastic modulus of the polymer films is probably due to a decrease in the chemical network density. The glass transition temperatures of the epoxy polymer materials were determined by differential scanning calorimetry. With AD-1, the dependence of the glass transition temperature of the epoxy materials on the diluent content also passes through a minimum. On introducing up to 10% AD-1, the glass transition temperature decreases, and with a further increase in the AD-1 content it increases, which well agrees with the results of physicomechanical tests (Figs. 7a, 7c, curves 1). Introduction of an active diluent leads to a decrease in the glass transition temperature of the cured coatings (Figs. 7a, 7c) irrespective of the structure of the oligomer used. For the compounds based on EO-2, an increase in the active diluent content from 5 to 15% does not influence the glass transition temperature of the samples irrespective of the kind of the active diluent. The least pronounced decrease in the glass transition temperature is observed with AD-3. Irrespective of the structure of the oligomer used, the most pronounced decrease in the glass transition temperature is observed in the systems with ACA-2 as curing agent (Figs. 7b, 7d). Thus, the structures of the active diluent and epoxy oligomer influence the dependence of the glass transition temperature of the cured materials on the active diluent content.
Influence of the active diluent content on the glass transition temperature of cured epoxy materials. (a) Coatings containing the epoxy oligomer based on 4,4′-isopropylidenediphenol, CeTePox 1312 FS H, and various active diluents: (1) alkyl glycidyl ether with С12–С14 alkyl fragment, (2) propylene glycol diglycidyl ether, and (3) glycidyl neodecanoate; (b) coatings containing the epoxy oligomer based on 4,4′-isopropylidenediphenol, glycidyl neodecanoate, and various curing agents: (1) CeTePox 1312 FS H, (2) CeTePox 1588 L H, and (3) ITAMINE CA54; (c) coatings containing the epoxy oligomer based on 4,4′-methylenediphenol, CeTePox 1312 FS H, and various active diluents: (1) alkyl glycidyl ether with С12–С14 alkyl fragment, (2) propylene glycol diglycidyl ether, and (3) glycidyl neodecanoate; (d) coatings containing the epoxy oligomer based on 4,4′-methylenediphenol, glycidyl neodecanoate, and various curing agents: (1) CeTePox 1312 FS H, (2) CeTePox 1588 L H, and (3) ITAMINE CA54.
CONCLUSIONS
Introduction of alkyl glycidyl ether with the С12–С14 alkyl fragment leads to the most pronounced decrease in the viscosity of epoxy compounds. The viscosity of epoxy compounds decreases to the greatest extent when introducing up to 10% active diluent. The decrease in the viscosity of the compounds is not additive, which is due to changes in the intermolecular interactions in the systems. Larger deviation from the additivity law is observed with monofunctional active diluents.
Introduction of the active diluent alters the course of curing of the epoxy compounds. With an increase in the active diluent content, the initial rate of the gel fraction buildup in the coatings remains constant, but the time before the network polymer starts to form increases from 5 to 30 min. This is caused by the formation of polymers that are washed out with acetone when using monofunctional diluents. This is also associated with lower reactivity of aliphatic epoxy oligomers and monofunctional compounds used as diluents. This leads to a decrease in the consumption rate of epoxy groups and hence to an increase in the time in which the network polymer starts to form.
Both the elastic modulus and the ultimate strength of epoxy polymer films decrease with an increase in the active diluent content. However, when using AD-1, the dependences of the ultimate strength and glass transition temperature on the active diluent content pass through minima. At up to 10% AD-1 content, the glass transition and ultimate strength of the epoxy films decrease because of a decrease in the chemical network thickness. At higher AD-1 content, the ultimate strength and glass transition temperature of the samples formed in the presence of AD-1 increase owing to the antiplasticization effect.
Compounds with up to 10% content of monofunctional diluents have acceptable level of rheological, elastic-strain, and thermal properties. With the bifunctional diluent, the acceptable set of properties is reached at the diluent content of up to 20%.
REFERENCES
Kim, J.-M., Kwak, E.-G., Lee, C.-H., and Lee, S.-K., Adv. Mater. Res., 2013, vol. 687, pp. 229–234. https://doi.org/10.4028/www.scientific.net/AMR.687.229
Khalid, N.H.A., Hussin, M.W., Ismail, M., Basar, N., Ismail, M.A., Lee, H.-S., and Mohamedet, A., Constr. Build. Mater., 2015, vol. 93, pp. 449–456. https://doi.org/10.1016/j.conbuildmat.2015.06.022
Yeon, K.S., Choi, Y.S., Kim, K.K., and Yeon, J.H., Constr. Build. Mater., 2017, vol. 140, pp. 336–343. https://doi.org/10.1016/j.conbuildmat.2017.02.116
Balas, A., Palka, G., Foks, J., and Janik, H., J. Appl. Polym. Sci., 1984, vol. 29, pp. 2261–2270. https://doi.org/10.1002/app.1984.070290702
Jomier, A., Amari, K., Bernquist, H., Glennstål, M., and Wasson, B., in Advances in a Coating Technology Conf., 2010, pp. 268–270.
Huang, S., Xiao, J., Zhu, Y., and Qu, J., Prog. Org. Coat., 2017, vol. 106, pp. 60–68. https://doi.org/10.1016/j.porgcoat.2017.02.011
Cui, C., Guo, X., Han, Z., Shi, J., Sun, Z., Duan, S., Liu, B., and Lin, Z., IOP Conf. Ser.: Earth Environ. Sci., 2019, vol. 252, p. 022055. http://doi.org/10.1088/1755-1315/252/2/02205
Zhang, G., Xie, Q., Ma, C., and Zhang, G., Prog. Org. Coat., 2018, vol. 117, pp. 29–34. https://doi.org/10.1016/j.porgcoat.2017.12.018
Duong, N.T., Hang, T.T.X., Nicolay, A., Paint, Y., and Olivier, M.-G., Prog. Org. Coat., 2016, vol. 101, pp. 331–341. https://doi.org/10.1016/j.porgcoat.2016.08.021
Baibaeva, S.T., Mirkind, L.A., Krylova, L.P., Navyazhskaya, E.A., and Salova, A.S., Metody analiza lakokrasochnykh materialov (Methods for Analysis of Paint-and-Varnish Materials), Moscow: Khimiya, 1974.
Karyakina, M.I., Ispytanie lakokrasochnykh materialov i pokrytii (Testing of Paint-and-Varnish Materials and Coatings), Moscow: Khimiya, 1998, pp. 75–80.
Irzhak, V.I., Korolev, G.V., and Solov’ev, M.E., Russ. Chem. Rev., 1997, vol. 66, pp. 167–186. https://doi.org/10.1070/RC1997v066n02ABEH000256
Irzhak, V.I., Vysokomol. Soedin., Ser. A, 2000, vol. 42, no. 8, pp. 1616–1632. https://doi.org/10.1070/RC1997v066n02ABEH000256
Moshinsky, L., Epoksidnye smoly i otverditeli (Epoxy Resins and Hardeners), Tel Aviv: Arcadia, 1995.
Funding
The study was financially supported by the Ministry of Science and Higher Education of the Russian Federation (agreement no. 05.607.21.0316, unique agreement identifier RFMEFI60719X0316).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kurbatov, V.G., Pugacheva, T.A., Malkov, G.V. et al. Effect of Active Diluents on Properties of Epoxy Compounds and Coatings Based on Them. Russ J Appl Chem 93, 1340–1348 (2020). https://doi.org/10.1134/S1070427220090050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427220090050