Abstract
The recently developed Ru containing drugs have some significant advantages over contemporary Pt-based agents due to their more sophisticated mechanisms of action, lower toxicity, absence of cross resistance, and broad spectrum of activities. In this review, we discuss the recent noteworthy entries devoted to Ru-based anticancer drugs, their industrial utilization and market value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Metal complexes are used intensively in pharmaceutical science due to a wide variety of their building blocks and types of interactions (pi-stacking, H-bond, spatial recognitions, coordinate bond etc.) leading to combination of rigidity around metal centers and flexibility of ligands structures [1]. The anti-cancer drug cis-platin was developed nearly 50 years ago, since then some of its analogues also reached clinical application [2]. Along with successful treatment of various types of cancer, these were characterized by severe side-effects—kidney damage, nausea and suppression of bone-marrow activity [3–6]. Besides the side-effects, such agents induced the drug resistance [8]. So, a new generation of drugs that metabolize readily on the way to their targets [11, 12] characterized by high uptake in the cells and sufficient drug concentration in the affected cells [9,10] were needed. In this respect, some transition metal-organic compounds aiming different targets have been developed [13–18].
Ruthenium organic compounds were determined to be the efficient alternative of Pt based drugs. Among the most important advantages of Ru(II) and Ru(III) complexes was their high activity against metastatic cancers. Such compounds could alter their oxidation states inside the cells. Both Ru(II) and Ru(III) can adopt hexacoordinated octahedral configuration where additional axial ligands could help to tune the steric and electronic properties of the complexes due to relatively low energy barrier for interconversion, which resulted in less severe and fewer side effects those of their Pt analogues. A number of Ru complexes can modulate redox properties of a system and ligand interactions with various ancillary ligands [20, 21].
Conclusively, the second major area of application of Ru complexes is their exclusive catalytic activity discovery of which was distinguished by the Nobel Prize award. Catalytic applications of such complexes cover a broad variety of chemical transformations, that were developed and adopted on the industrial scale. Contribution of Ru complexes in preservation of fossil resources is of high importance. The Ru complexes catalysis used in oleo chemistry and related production of value-added synthetic olefins, polymer additives, surface coatings, and pharmaceuticals from natural seed oil feedstock is available in many countries. Fluorescence of some ruthenium complexes is quenched by oxygen and is used in oxygen sensors. Ruthenium red, [(NH3)5Ru–O–Ru(NH3)4–O–Ru(NH3)5]6+, is a biological marker used to stain polyanionic molecules such as pectin and nucleic acids in light and electron microscopy. Thus, the aim of this review is to expose information on recently reported Ru-based complexes of considerable importance for medical purposes. This presentation also covers the retrospective approach to development and industrial applications of various Ru-based complexes. This review is not meant to be comprehensive but rather a snapshot of the particular rapidly changing scientific and industrial areas. We hope that the presented below information would prove to be useful for newcomers to the field as well as those already familiar with the subject under consideration.
Historical Background
The precious metals were not only precious in value but also in medical applications. In ancient India and China, around 3500 years ago, precious metals have been used for medical purposes as those were believed to cure various diseases due to their rarity in earth. Since then the proper mechanism of these metals complexes action still remains somewhat unclear. Nonetheless, researchers around the globe are trying to upgrade their design, enrich potency and reduce side-effects of the complexes.
Since the arrival of cis-platin for clinical trials in 1979 [22], search for alternate metal-based drugs against cancer was stimulated and paved the way to targeted chemotherapy and addressed specific cancer physiology [14, 23]. As it was determined recently, Ru analogues of cis-platin were less toxic, which stimulated coordination chemistry of Ru to grow rapidly. The abundant report on the enhanced in vitro and in vivo activity of Ru complexes and remarkably successful clinical trials of Ru based anti-cancer drugs NAMI-A, KP1019, and KP1339 (although none in clinical uses) initiated a lot of researches working in this area [24].
Bergamo and Sava speculated in 2011 that some of the mentioned above problems might be tackled using a ruthenium substitute [25]. Yet to be sanctioned for medical use as anti-cancer drugs, Ru complexes have clear advantages over the contemporary Pt based drugs due to many reasons discussed in literature [26]. Cytotoxic potency of the Ru-complexes was consistent with their hydrophobicity and cellular uptake properties, that were of 2-fold value of that of cis-platin in 2D A549R cancer cells and 3D multi-cellular A549R tumor spheroids, as stated by Chao et al [27].
Recently developed drugs based on Ru-complexes. Fluorine can impart specific properties to drugs by enhancing their lipophilicity and biological activity [28–30]. Some candidates of the kind have been produced including those that chelated ruthenium with drug-like heterocyclic small molecules containing the trifluoromethyl group [31].
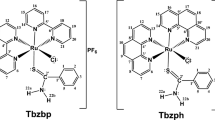
Ruthenium can ligate not only with 1,10-phenanthroline (phen) forming polypyridyl complexes but also with 2-phenylimidazo[4,5-f] [1,10] phenanthroline (PIP) and its derivatives producing Ru(phen)2(PIP)2+, which is a well-known mitochondria-targeting drug [32]. The C–N coordination site of 7,8-benzoquinoline (bq) ligand can reduce the positive charge of Ru center thus increasing cellular uptake [33]. However, fixation of Ru as an efficient drug producing metal can result not only in the cellular-uptake but lipophilicity, membrane-permeability and bio-availability. For this reason substitution of the NH-function in PIP with tert-butylbenzene and attachment of trifluoromethyl group were necessary. Recently, improvement of bio-activity and physicochemical properties of the complexes by structural modifications were studied by Chao et al [27]. Among the Ru(II) complexes presented in Fig. 1, the fourth one displayed higher potency than that of its Pt counterpart according to the vitro cytotoxicity tests. The authors also determined 3D multi-cellular tumor spheroids based on A549R cells, and this model was tested for in vitro activity of complex 4 toward multidrug-resistant (A549R) tumor cells. The complex 4 could efficiently induce A549R cell apoptosis via multiple pathways [27].
Chemical structures of Ru(II) complexes 1–4 [27].
Heterodinuclear Ru(II)–Pt(II) complex. Apart from the regular homo dinuclear [(Ru(bpy)2)2(1-dpp)]4+ or mononuclear [Ru(bpy)2(dpp)]2+ Ru complexes, application of heterodinuclear complexes application of bridging ligands such as ([Ru(bpy)2(1-dpp)PtCl2]2+, bpy = 2,20-bipyridine, dpp = 2,3-bis(2-pyridyl)pyrazine) was preferable due to their ability to stabilize more efficiently the triplet metal-to-ligand charge transfer (MLCT) excited states. Coupling of Ru-based chromospheres with cis-platin has been tested [34] and the systems demonstrated not only enhanced DNA binding but also provided light absorbing capability [35–37]. Mononuclear and heterodinuclear Ru(II) complexes demonstrated remarkable DNA-binding properties. According to Nakanayashi et al. [36, 38], mononuclear Ru(II) complex [Ru(tBu2bpy)2(dpp)]2+ and heterodinuclear ruthenium(II)–platinum(II) complex [Ru(tBu2bpy)2(l-dpp)PtX2]2+ (X = Cl (1), Br (2), I (3)) and [Ru(bpy)2(l-dpp)PtCl2]2+ (4) combined with complexes of CT-DNA and DNA demonstrated photo cleavage using pBR322 supercoiled plasmid DNA upon irradiation by visible light and their cytotoxicity against HeLa cervical cancer cell line. As the complexes were cleavage inactive in darkness, photoinduced DNA cleavage was observed. DNA photocleavage of complexes 1–3 proceeded via photoredox pathway, while singlet oxygen pathway was observed for the [Ru(tBu2bpy)2(dpp)]2+ complex. Similar to cis-platin, in presence of light cytotoxicity was determined for this complex, along with 2 and 3. The order of such cytotoxicity of 1-4 could be lined as following: 2 > 3 > 1 ≈ 4 which could probably be explained in terms of lipophilicity. DNA binding ability of the complexes followed the order 1 > 4 > 3 ≈ 2 > [Ru(tBu2bpy)2(dpp)]2+.
Macrocyclic Ru(III) complex. Among Rucomplexes that have potential of in vitro and in vivo anti-cancer activities [34, 39], NAMI-A or (ImH)[trans-Ru(III)(Im)(DMSO)Cl4] deserves special attention due to its ability of cell adhesion [41]. It is non-cytotoxic towards solid tumors but acts against tumor metastases and angiogenesis [40]. It was determined that substitution of the axial ligand had the major effect on the cellular uptake and hydrolytic stability. For example, KP-1019 (InH [Ru(III)(In)2Cl4]) complex, where In = indazole, demonstrated some promising features [42]. Its shift from the parent complex led to alteration of its anti-colorectal carcinoma activity while the latter was cis-platin resistant and did not demonstrate specific anti-metastasis activity [43].
Sadler and Dawson [44] developed the “Piano-Stool” RM and RAPTA complexes. RM complex contained ethylenediamino ligand and exhibited in vitro cytotoxicity [45], whereas the RAPTA-type product contained phosphoadamatane anti-tumor ligand [46]. Another complex RDC11 ([Ru(II)(Phen)(CN)(CH3CN)2]PF6) demonstrated in vitro and in vivo activities against several human cancer cell lines [47].
The macrocyclic compound [Ru(III)–(N2O2)Cl2]Cl exhibited anti-tumor activity [48] and inhibited the processes of angiogenic endothelial cell tube formation and cancer cell invasion, however it was relatively non-cytotoxic towards in vitro endothelial and cancer cell lines. It distinctively suppressed the expression of vascular endothelial growth factor receptor-2 (VEGFR2) and the associated downstream signaling that was vital for tumor angiogenesis. The latter effect was not exhibited by the other Ru based anti-cancer complexes.
Arene–ruthenium(II) complex. Not only Ru-complexes challenged the cis-platin in pharmaceutical industry but also Fe-complexes that could pass through the cell membrane. The recent discovery of different pathways of smuggling Ru(II) by transferrin has been described [49]. It was determined that anti-tumor Ru(III) complexes were able to replace Fe(III) in transferrin. Interestingly, the Ru-arene bond could be conserved during hydrolysis while part of the coordination environment of Ru(II) remained unchanged. This property can save certain ligands from the detoxification or deactivation in biological fluids. However, these properties have not yet been fully explored by conjugating metal centers with the particular kinds of ligands. However, study of novel arene-Ru(II) complexes with structural analogues of curcumin [50] characterized by anti-oxidant properties, would have led to anti-cancer, anti-tumor, anti-angiogenic, and anti-inflammatory activity of the products [51].
Some late strategies involved bioactive chelating ligands [52], such as avobenzene (1-(4-tertbutylphenyl)-3-(4-methoxyphenyl) propane-1,3-dione, AVBH), which is used in sunscreen and cosmetic products as an oil soluble UVA filter [53]. It absorbs UV rays that in turn get converted into heat and dissipated in the skin. Moreover, this effect has also shown promising anti-cancer activity having similar efficiency with doxorubicin against chemoresistant cell types [54]. The latter is the known DNA intercalator disrupting topoisomerase II mediated DNA repair [55]. It also generates free radicals and causes damage to cellular membranes, DNA, and proteins, that can be explored for the action of AVBH in cancer cells. Ru(II)–AVBH complex was designed for activity enhancement and cellular uptake improvement. It was highly hydrophilic and upon introduction of PTA or PTA-Me as co-ligands, its activity could be tuned. Pettinari et al. [56] suggested testing of such complexes for their cytotoxicity against human ovarian carcinoma cells, A2780 and A2780-cis-R as well as non-tumor Human Embryonic Kidney (HEK293) cells.
Oligonuclearpolypyridylruthenium(II) complexes. Action of Ru complexes as anti-microbial agents is among the actively studied areas [57–59]. First reported by Dwyer et al [57], the mononuclear complex containing polypyridyl ligands demonstrated effectiveness against both gram-positive and gram-negative bacteria, although their high potential has been determined against drug-susceptible strains, particularly gram-positive bacteria and to a lesser extent against some current drug-resistant strains [60]. The mononuclear complexes have been surpassed by oligonuclear, and di-, tri-, tetra- nuclear complexes in terms of anti-microbial potency. For example, polypyridyl–Ru(II) complexes demonstrated excellent activity against two gram-positive strains of S. aureas (one susceptible and another MRSA). These contained bis[4-(4′-methyl-2,2′-bipyridyl)]-1,n-alkane ligand (bbn) bound with Ru center. However, variable activity was found against E. coli and P. aeruginosa (both are gram-negative) [61]. As per preliminary studies, the di-nuclear complexes were not highly toxic to human cells. However, further studies should be carried out against a wider range of bacteria and particularly pathogens, that are included in ESKAPE criteria [61].
Any antibacterial agent should have lower toxicity against human and/or animal cells than bacteria. The positively charged Ru complexes would be more prone to bacterial cell walls than the eukaryotic counterparts due to the presence of more negatively charged components in the former cell walls. The eukaryote counterpart is characterized by high content of zwitterionic phosphatidylcholine in the outer membrane leaflet providing lesser scope for electrostatic interactions [62].
Ru(II)–naphthoquinone. Characterized as antimicrobial [63, 64], antiparasitic [65], antiviral [66], and anticancer [67] agents naphthoquinones (NQs) can be found in plants either in reduced or in glycosidic form, or as secondary metabolites in actinomycetes, fungi, lichens, and algae [68]. They can generate reactive oxygen species (ROS) such as H2O2, superoxide anion radical (O2•–) and the hydroxyl radical (•OH) [68] that in turn can lead to apoptosis via the extrinsic and intrinsic mitochondrial pathways [61]. Menadione thioanalogues NSC 95397 (2,3-bis[2-hydroxyethylthio]-1,4-naphthoquinone) and Cpd 5 (2-(2-mercaptoethanol)-3-methyl-1,4-naphthoquinone) are two examples of inhibitors of CDC25 phosphatase which is an important enzyme controlling transition of cells through the cell cycle by dephosphorylation of cycle independent kinases [69].
Another important 1,4-NQ derivative is lapachol (2-hydroxy-3-(3-methylbut-2-en-1-yl) naphthalene- 1,4-dione) that can act as O,O-bidentate anionic ligand and has intrinsic anti-cancer properties. It can be included in design of hybrid organic/inorganic anti-cancer agents for enhancing their potential as chelating ligands in Ru(cym) complexes [70]. Antitumor activity could be improved by modulating redox properties of Ru(II), Os(II), and Rh(III) complexes with activity in low micromolar ranges in several cancer cell lines. However, higher ROS-induced apoptosis and cell cycle arrest was determined for Ru-bio active ligands rather than their Os/Rh-Lapachol complexes. It could be induced by the synergistic effect of Ru with the ligands [52].
Benzene–Ru(II) complex. Arene–Ru(II) complexes adopt a distinct three-legged piano-stool conformation in which the arene takes the seat and chelating ligands along with the auxiliary ligands make the stand of the piano stool thus possessing lipophilic and hydrophilic properties [71]. Such structures demonstrate aqueous solubility with adequate lipophilicity for crossing cell membranes. Although the arene stabilizes Ru(II), there are scopes for combinations of substituents that can tune the properties of the complexes. Most importantly, there are similarities between well tested Pt-complex and Ru-complexs in case of ligand exchange kinetics crucial for anti-cancer activity [72]. In turn it involves the cytotoxicity mechanism to proceed via hydrolysis of the Ru–X bond producing active species [73].
Arene ruthenium complexes of the type [Ru(η6-arene)(PTA)Cl2] (PTA = 1,3,5-triaza-7-phosphaadamantane) [74], (η6-arene)RuCl2(imidazole) [75], (η6-arene)RuCl2(DMSO) [76], [Ru(η6-arene)(YZ)Cl]· [PF6][77] (YZ = chelating diamine) as well as dinuclear compounds [78], tri [79] and tetranuclear clusters [80] such as [H3Ru3(η6-C6H6)(η6-C6Me6)2O]+ and [H4Ru4(η6-C6H6)4]2+ have been studied in vitro and in vivo for their antitumor activity. These complexes targeted DNA, and their interactions with proteins were determined [74, 81]. Scientists are still in active search of complexes that target both aforementioned sites [72, 81].
Ramesh et al. [85] indicated potential of Ru(II)–benzene complexes comprising aroylhydrazone ligand against human cancer cell lines. Aroylhydrazones are characterized by amide-imidol tautomerism that can result in their ability to bind with the metal multiple sites depending upon the reactions conditions, nature of the metal ion and some more factors. They coordinate via azomethine N being in neutral amide form and produce five-member chelate rings with bidentate N,O donor ligands in monobasic imidol form [83]. The azomethine group bears biological importance due to presence of a lone pair of electrons in sp or sp2 hybridized orbitals of trigonal hybrid N atom [84].
Cyclometalated Ru(II) complexes. Ru-Carbon σ bond in cyclometalated Ru(II) complexes can lower the redox potential of Ru(III/II) couple significantly. Accordingly, they can be used as good catalysts for generating hydroxyl radicals in the Fenton-like reactions. Also, good lipophilicity supports their penetration inside cells [33]. Many cancer cells demonstrate low levels of catalase activity [88] prompting Pfeffer and co-workers [33, 86] and Chao and colleagues [87] to use the complexes in anti-cancer drugs.
Anti-cancer activity of the complexes [Ru(bpy)2(C^N)]Cl {bpy = 2,2-bipyridine, C^N = deprotonated cyclometalating ligand (2-phenylpyridine (phpy)} against tumors L1210 and HeLa and non-tumor BALB/3T3 clone A31 cell lines using calf thymus DNA (CT–DNA) indicated these complexes as DNA minor groove binders and/or intercalators. Cytotoxicity against L1210 and HeLa was higher than that of cis-platin contrary to their action against BALB/3T3 clone A31 [89].
Ruthenium(II) amino acid complexes. Ru–amino acid coordinated complexes demonstrated their potential against murine breast cancer MDA-MB-231 [90, 91]. Five Ru-amino acid complexes namely [Ru(AA)(bipy)(dppb)]PF6, where AA = methionine, glycine, leucine, aspartic acid, or alanine bipy = 2,29-bipyridine; and dppb = [1,4-bis(diphenylphosphine)butane] were tested against S180 murine sarcoma cell line in vitro. The [Ru(gly)(bipy)(dppb)]PF6 was found to be the most active against S180 cells with low cytotoxicity against L929 cells [92].
DNA mismatches repair application. Ru-complexes may also be used for selective, signal-on-probes for DNA mismatches. Barton et al [94] found molecular “light switches” for duplex DNA in the derivatives of [Ru(bpy)2dppz)]2+ (dppz = dipyridophenazine). Accordingly, the compound could be used as a structural probe, cellular imaging device as well as developing cytotoxic and photoactive molecules [96–98]. The striking feature came from the intermolecular hydrogen bonding formation with water molecules which caused quenching in luminescence when neither H-bonding interaction nor quenching occurred in aprotic solvents. Upon intercalation with well-matched DNA, they luminesce brightly due to protection of dppz ligand from aqueous medium [94, 95].
Crystal structure of the complex bound to an oligonucleotide duplex containing a mismatch revealed that, similarly to rhodium metalloinsertors, the ruthenium complex bonded at the mismatch site in the minor groove by metalloinsertion [100]. The complex [Ru–(bpy)2dppz]2+ is not mismatch-specific, however it readily binds to well-matched sites in DNA duplex via intercalation and exhibits brighter emission in DNA-mismatch sites rather than well-matched DNA [99]. Ruthenium complexes with expansive inserting ligands like 5,6-chrysenequinone diimmine (chrysi) demonstrated mismatch specificity in binding but did not luminesce at ambient temperature [101]. Recently Barton et al. [93] explored models of the complex bound to well-matched and mismatched sites. Using the DNA coordinates of the crystal structure of Δ-[Rh(bpy)2(chrysi)]3+ bound by metalloinsertion to an AC mismatch [102–106] they oriented Δ-[Ru(Me4phen)2dppz]2+ into the mismatch site from the minor groove while minimizing steric clashes with the Me4phen ancillary ligands and DNA (Fig. 2).
Views down the helix axis of Δ-[Ru(Me4phen)2dppz]2+ modelled into the crystal structures of DNA duplexes. Ruthenium complex is presented green with nitrogen atoms in blue. (a) Metalloinsertion at a mismatch site from the minor groove; the extruded mismatched bases are presented orange. (b) Side-on intercalation at a well-matched site from the major groove [93].
Hypochlorous acid detection and imaging application. Apart from contemporary analytical methods such as HPLC [107], electrochemical method [108], electrophoresis [109], UV spectrophotometry [110], and fluorescent probes [111] can detect HClO. Fluoroscent probes are popular for their advantages in in vitro and in vivo analysis [112], though these are not free from complications. The transition metal complexes can be used as luminiscence probes and cellular chemosensors due to their superior photophysical properties such as high water solubility, intense polarized luminiscence, visible-light emission and absorption, high chemical and photostability, large stoke shift, low cytotoxicity, and long lifetime [113, 114].
The probes are generally designed upon conjugation of Ru along with HClO recognizing moieties such as nitrophenyl derivatives [115], phenothiazine [116], ferrocene [117], and oxime derivatives [118]. Luminescent probes for environmental and biological applications include Ru(II) with 3 diimine ligands such as 2,2′-bipyridine (bpy), 1,10-phenanthroline (phen) and bathophenanthroline [119–122].
Visible light photoredox catalysis. Application of visible wavelengths rather than UV has certain advantages providing the green method avoiding deterioration of products and side reactants. Such approach became an important tool in preparing complex molecules as well as natural products like heitziamide A [104] and aplyviolene [105].
Ru Complexes such as tris(2,2′-bipyridine) ruthenium(II) (Ru(bpy)32+) (Fig. 3) [102, 103] have been used widely, although in some instances Ru and Ir have been substituted by other metals successfully [106].
Structure of Ru(bpy)32+ and its oxidative and reductive quenching cycles [94].
Application in Photodynamic Therapy
Another promising selective cancer treatment procedure is PDT (Photo Dynamic Therapy), in which reactive oxygenated species such as cytotoxic singlet oxygen 1O2 are generated by irradiating a non-toxic photosensitizer (PS) under specific wavelengths. Depending upon oxygen available in the medium, it is believed to be the major cytotoxic agent [118] of high efficiency [119].
For the relatively long-living triplet metal in ligand charge transfer excited states (MLCT), Ru(II) complexes with arene [77, 123], porphyrin [124] and polypyridyl [124, 125] along with tris(bipyridyl) ruthenium(II) have proven to act as photosensitizers for PDT [126, 127] and nucleic acid probes [128, 129]. The latter complex characterized by synthetic tailor ability, high thermodynamic stability, long lifetime of MLCT excited state [130], non-linear optical properties [131], and potential two-photon absorption properties (TPA) gave rise to self-applications of O2 sensing [132, 133] and photodynamic therapy [134, 135].
Biomolecule detection and protein staining application. Luminescent cyclometalated Ir(II) complexes containing 2-phenylpyridine (ppy) ligands [136] and cyclometalated Pt(II) complexes containing 2-phenylpyridine (ppy) ligands [137] are used for protein detection and staining. On the other hand, Ru(II) and Os(II)-complexes of the general formula [MII(N^N)(X)3(L)]n+, where (X)3 are facial or meridional tridentate ligands, and N^N are bpy-like aromatic di-imines [138] with [MII(N^N)X)3(L)]n+ core could be used as luminescent switch-on/off probes when bonded with biomolecules [139]. Although commercially available SYPRO ruby dye [140] is used widely, but its undisclosed composition retards its research progress.
Aβ Aggregation inhibitors. Among chemical approaches to tackling Alzheimer disease [136–141], Pt-chelates [142], cyclometalated Pt complexes [143], binuclear Ru(II)-Pt(II) complexes [145], planar aromatic C–N co-ligands containing Ir and Rh complexes [146], and Ir/Pt/Ru-benzimidazole [147] complexes were synthesised for reduction of Aβ neurotixicity. Many anti-Aβ aggregation complexes of Fe(II) and Ni(II) [148] are known as bulky, saturated, non-phenanthroline based, triple helical, and metallo-supramolecular. Among various types of metal complexes that target the very aggregation [149] is Ru–Pmru20 complex, that demonstrates high anti-cancer activity [150–155].
Electrochemical sensor application. Importance of Ru complexes and oxides in this area is about modification of electrodes for electro analysis of the compounds that possess electrocatalytic activity [156]. For electrode modification and electrocatalysis, they are pure favourites for being able to undergo a reversible redox process. Ru-complexes support simultaneous determination of PA, 6-TG and catecholamines at multiwalled carbon nanotube modified carbon paste electrode with wider linear range and low detection limit [157, 158].
PA has many chemical and medical applications; it is a heavy metal chelator used against Wilson disease [152], cystinuria [152], rheumatoi arthritis [153], and systemic sclerosis [154]. The 6-thioguanine (6-TG) on the other hand, is an antimetabolite which was used as cytostatic agent in chemotherapy for treating acute lymphoblastic leukaemia in children [159]. This compound belongs to thiopurine family which also treats ulcerative colitis (UC), a form of Inflammatory Bowel Disease (IBD) and some autoimmune diseases along with anticancer and anti-tumour activities. Both of them are sulphur containing drugs listed in the WHO essential medicines [160]. Catecholamine and its derivatives are produced by nerve tissues (including the brain) and adrenaline gland. Its analogues are most abundant in the human body in the form of dopamine (DA), norepinephrine (NE) and epinephrine (EP) [161]. In the diagnosis, it is used as stimulant drugs, neuroblastoma and so on. In pheochromocytoma, CA levels in urine and blood are determined. By determining CA, many electrochemical sensors are also being devised [162].
Antihypertension drugs. Stable ruthenium(II) catalysts [163, 164] play a significant role in synthesis of nonpeptidic angiotensin II receptor blockers (ARBs), that are efficient antihypertensive drugs [160]. In this context, researcher involved bifunctional ligands in the C–H bond activation of C5-aryl-substituted tetrazoles to get the targeted results [165]. Specifically, carboxylates [166] and phosphinous acids [167] support efficient C–H arylation of aryltetrazoles [168], whereas phosphate and sulfonate additives can be subsequently utilized [169].
Significant rate acceleration by amino acid ligands [170, 171] in ruthenium catalyzed remote alkylation [172] has been determined. Recently, Ackermann et al [173] reported the practical approach to important in medicinal chemistry and pharmaceutical industry biaryl-substituted tetrazoles that involved ruthenium(II) amino acid catalysts for direct C–H arylation of 5-aryltetrazoles highlighting the unique long-term efficiency of ruthenium amino acid catalysts in C–H activation chemistry (Fig. 4). Here formal oxidative addition of aryl chloride 2 by a SET-type process to Ru(II) species gave complex 6, reductive elimination of which led to product 3 along with regeneration of the active catalyst 4.
The proposed catalytic cycle for C–H arylation [172].
Dye-sensitized solar cells. Ruthenium complexes were used to sensitize transparent electrodes for nearly 30 years, due to their wide absorption spectrum [174]. The most popular complexes are N719 (red dye) [Ru(dcbpy)2(NCS)2][TBA]2 (where dcbpy = 4,4′- dicarboxy-2,2′-bipyridine and TBA = tetrabutylammonium) and N749 (black dye) [Ru(Htctpy) (NCS)3][TBA]3 (where tctpy = 4,4′,4″-tricarboxy-2,2′:6′,2″-terpyridine) [170]. However, the limitation of such Ru(II) complexes is coursed by presence of thiocyanate ancillary ligands [175].
Dye-sensitized solar cells (DSSCs) that used these dyes and electrolyte solutions containing the I−/I3− redox couple, demonstrated quantum efficiency and the highest energy conversion efficiency [176]. Prior to that study in 2003, efficiency of DSSCs using Ru complex dyes and Co complex redox couples was determined to be as low as 4% [177]. The efficiency was much lower than that of DSSCs using Ru complex dye and the I−/I3− redox couple. The lower efficiency was partially due to faster charge recombination between injected electrons and the Co complex [178]. In 2010, it was demonstrated that organic dyes with bulky peripheral units were able to retard the charge recombination with Co complex redox couples; that is, the bulky units could block the Co complex approach of TiO2 surface [179]. In 2014, DSSCs employing a porphyrin dye in conjunction with Co complex redox couple achieved higher efficiency than the DSSCs with a Ru complex dye and the I−/I3− redox couple [180].
In 2013, Frey et al [181] indicated the comparable efficiency for a Co complex and the I−/I3− bipyridine coordinated Ru complex dyes. While the efficiency of 8.6% was achieved with combination of Ru complex dye and Co complex redox couple, the external quantum efficiency was ca 70% and the open circuit voltage (Voc) was not as high as the value expected from the redox potential of the Co complex. In 2014, efficiency of 8.7% and ~80% IPCE was reported for DSSCs using a SCN-free Ru complex with long alkyl chains and Co complex redox couple [182]. However, the reason was not clear since many dyes, except for Ru dyes, demonstrated ~90% IPCE in DSSCs using a Co complex redox couple [183]. The low IPCE observed from most of Ru complex dyes with Co complex redox couples prompted that Ru complexes could have some intrinsic problems with Co complex redox couples. While the quantum efficiency of the DSSCs upon using Ru complex dye and Co complex redox couples cannot be explained entirely by electron recombination, somehow this point has not been addressed explicitly. Meanwhile, the reason for low efficiency for the DSSCs using Ru and Co complexes was examined with DFT calculations that indicated partial electrostatic interactions between Ru complex and Co complex [184]. Since the negatively charged SCN ligands are commonly used for Ru complex dyes, a possible reason for high efficiency of SCN-free Ru complex dye [181] could be due to lower electrostatic interactions with Co complexes. Chou et al [185] reported highly efficient SCN-free terpyridine-coordinated Ru complex dye. Their motivation was to increase stability of Ru complex dye in conjunction with the I−/I3− redox couples.
CONCLUSIONS
Ruthenium anticancer agents have recently been efficiently introduced in clinic due to their promising activity on resistant tumours. Activity of ruthenium compounds depends on both the oxidation state and the nature of ligands. Ruthenium-centred antimalarial, antibiotic and immunosuppressive drugs have been developed.
Low toxicity is one of important factors for application of ruthenium complexes as drugs in clinical practice. Ruthenium can mimic the binding of iron to biomolecules exploiting the mechanisms that body has evolved for nontoxic transport of iron. Redox potential between different accessible oxidation states of ruthenium enables the body to catalyse oxidation and reduction reactions, depending on the physiological environment.
An important aspect of industrial application of Ru complexes is removal of ruthenium residues, mandatory in areas where the level of this heavy metal in the final products like pharmaceuticals, specialty polymers designed for food supplies, biomedical, textile and electronic applications could be problematic for human health. Overall, the high potential and versatility of Ru complexes attest their importance in biology, chemistry and some technical areas.
ACKNOWLEDGEMENT
Dr. Roy is thankful to Netaji Subhas Open University for financial assistance (Project Memo No: AC/140/2021-22 dated 01/11/2021) and computational facilities to write the present review.
REFERENCES
Hambley, T.W., Science, 2007, vol. 318, no. 5855, p. 1392. https://doi.org/10.1126/science.1150504
Wilson, J.J. and Lippard, S.J., Chem. Rev., 2014, vol. 114, no. 8, p. 4470. https://doi.org/10.1021/cr4004314
Munkarah, A.R. and Coleman, R.L., Gynecol. Oncol., 2004 vol. 95, no. 2, p. 273. https://doi.org/10.1016/j.ygyno.2004.09.018
Böhm, S., Oriana, S., Spatti, G., Di Re, F., Breasciani, G., Pirovano, C., Grosso, I., Martini, C., Caraceni, A., Pilotti, S., and Zunino, F., Oncology, 1999, vol. 57, p. 115. https://doi.org/10.1159/000012017
McGuire, W.P., Hoskins, W.J., Brady, B.F., Kucera, P.R., Partridge, E.E., Look, K.Y., Clarke-Pearson, D.L., and Davidson, M., New Eng. J. Med., 1996, vol. 334, no. 4, p. 1. https://doi.org/10.1056/NEJM199601043340101
Jamieson, E.R. and Lippard, S.J., Chem. Rev., 1999, vol. 99, no. 9, p. 2467. https://doi.org/10.1021/cr980421n
Galluzzi, L., Senovilla, L., Vitale, I., Michels, J., Martins, I., Kepp, O., Castedo, M. and Kroemer, G., Oncogene, 2012, vol. 31, no. 15, p. 1869. https://doi.org/10.1038/onc.2011.384
Kathawala, R.J., Gupta, P., Ashby, C.R. and Chen, Z.-S., Drug Resist. Updates, 2015, vol. 18, p. 1. https://doi.org/10.1016/j.drup.2014.11.002
Cossa, G., Gatti, L., Zunino, F., and Perego, P., Curr. Med. Chem., 2009, vol. 16, no. 19, p. 2355. https://doi.org/10.2174/092986709788682083
van Rijt, S.H., Mukherjee, A., Pizarro, A.M., and Sadler, P.J., J. Med Chem., 2009, vol. 53, no. 2, p. 840. https://doi.org/10.1021/jm901556u
Leung, C.-H., Zhong, H.-J., Chan, D.S.-H., and Ma, D.-L., Coord. Chem. Rev., 2013, vol. 257, nos. 11–12, p. 1764. doi 10.1016/j.ccr.2013.01.
Leung, C.-H., Zhong, H.-J., Yang, H., Cheng, Z., Shiu-Hin Chan, D., Pui-Yan Ma, V., Ruben, A., Chun-Yuen, W., and Dik-Lung, Ma, Angew. Chem. Int. Ed., 2012, vol. 51, no. 36, p. 9010. https://doi.org/10.1002/anie.201202937
Dik-Lung, Ma, Li-Juan, L., Ka-Ho, L., Chen, Y.-T., Zhong, H.-J., Daniel, Shiu-Hin Chan, Hui-Min, D.W., and Leung, C.-H., Angew. Chem., 2014, vol. 126, no. 35, p. 9332. https://doi.org/10.1002/ange.201404686
Muhammad, N. and Guo, Z., Curr. Opin. Chem. Biol., 2014, vol. 19, no. 4, p. 144. https://doi.org/10.1016/j.cbpa.2014.02.003
Ran, Xu., Zhao, Y., Liu, L., Longchuan, B., Yang, C.-Y., Zhou, B., Jennifer, L.M., Chinnaswamy, K., Jeanne, A.S., and Wang, S., J. Med. Chem., 2015, vol. 58, no. 12, p. 4927. https://doi.org/10.1021/acs.jmedchem.5b00613
Liu, Li.-J., Bingyong, He., Jennifer, A., Miles, W.W., Zhifeng, Mao., Weng, I.C., Lu, J.-J., Chen, X.-P., Wilson, A.J., Ma, D.-Lu. and Leung, Ch.-H., Oncotarget, 2016, vol. 7, no.12, p. 13965.
Liu, Z., Romero-Canelón, I., Qamar, B., Jessica, M.H., Habtemariam, A., Nicolas, P.E., Barry Pizarro, A.M., Guy, J.C., and Peter, J.S., Angew. Chem. Int. Ed., 2014, vol. 53, no. 15, p. 3941. https://doi.org/10.1002/anie.201311161
Leung, C.H., Lin, S., Zhong, H.-J., and Ma, D.-L., Chem. Sci., 2014, vol. 6, no. 2, p. 871. https://doi.org/10.1039/c4sc03094j
Strasser, S., Pump, E., Fischer, R.C., and Slugovc, C., Monatsh. Chem., 2015, vol. 146, p. 1143. https://doi.org/10.1007/s00706-015-1484-x
Gunanathan, C. and Milstein, D., Chem. Rev., 2014, vol. 114, no. 24, p. 12024. https://doi.org/10.1021/cr5002782
Tönnemann, J., Scopelliti, R., and Severin, K., Eur. J. Inorg. Chem., 2014, vol. 2014, no. 26, p. 4287. https://doi.org/10.1002/ejic.201402583
Clarke, M.J., Bitler, S., Rennert, D., Buchbinder, M., and Kelman, A.D., J. Inorg. Biochem., 1980, vol. 12, no. 1, p. 79. https://doi.org/10.1007/BF00769733
Medici, S., Peana, M., Nurchi, V.M., Lachowicz, J.I., Crisponi, G., and Zoroddu, M.A., Coord. Chem. Rev., 2015, vol. 284, p. 329. https://doi.org/10.1016/j.ccr.2014.08.002
Bergamo, A. and Sava, G., Chem. Soc. Rev., 2015, vol. 44, p. 8818. https://doi.org/10.1039/C5CS00134J
Bergamo, A. and Sava, G., Dalton Tran., 2011, vol. 40, no. 31, p. 7817.
Amin, A., and Buratovich, M., Mini-Rev. Med. Chem., 2009, vol. 9, no. 13, p. 1489. https://doi.org/10.1039/C0DT01816C
Leli, Z., Yu, Ch., Jiangping, L., Huaiyi, H., Ruilin, G., Liangnian, J., and Hui, C., Sci. Rep., 2016, article 19449. https://doi.org/10.1038/srep19449
Hagmann, W.K., J. Med. Chem., 2008, vol. 51, no. 15, p. 4359. https://doi.org/10.1021/jm800219f
Purser, S., Moore, P.R., Swallow, S., and Gouverneur, V., Chem. Soc. Rev., 2008, vol. 37, p. 320. https://doi.org/10.1039/B610213C
Müller, K., Faeh, C., and Diederich, F., Science, 2007, vol. 317, no. 5846, p. 1881. https://doi.org/10.1126/science.1131943
Takayoshi, S., Nobusuke, M., Masashige, B., Yukihiro, I., Ayako, M., Masaki, R., Yosuke, O., Hidehiko, N., Shinsuke, I., Katsuhiko, S., and Naoki, M., Chem. Med. Chem., 2014, vol. 9, no. 3, p. 657. https://doi.org/10.1002/cmdc.201300414
Qi, C., Yan, X., Huang, C., Melerzanov, A., and Du, Y., Potein Cell., 2015, vol. 6, p. 638. https://doi.org/10.1007/s13238-015-0179-8
Fang, M., Machalaba, N., and Delgado, R.A.S., Dalton Trans., 2011, vol. 40, p. 10621. https://doi.org/10.1039/C1DT10801hH
Vivian Wing-Wah, Yam., Vicky Wing-Man, Lee., and Kung-Kai, Cheung., Organometallics, 1997, vol. 16, no. 13, p. 2833. https://doi.org/10.1021/om961058i
Jain, A., Winkel, B.S.J., and Brewer, K.J., J. Inorg. Biochem., 2007, vol. 101, no. 10, p. 1525. https://doi.org/10.1016/j.jinorgbio.2007.06.036
Srinivas, G., Ravi Kumar, V., Laxma Reddy, Y., Praveen, K., and Satyanarayana, S., Russ. J. Gen. Chem., 2018, vol. 88, no.12, p.2621. https://doi.org/10.1134/S1070363218120253
Pakal’nis, V.V., Borovitov, M.E., Balova, I.A., Tunik, S.P., Ivanova, N.V., and Sizova, O.V., Russ. J. Gen. Chem., 2008, vol. 78, no. 8, p. 1594. https://doi.org/10.1134/S1070363208080227
Takakazu, Y., Shota, H., Misaki, N., and Yasuo, N., Inorg. Chim. Acta, 2017, vol. 454, p. 162. https://doi.org/10.1016/j.ica.2016.04.011
Clarke, M.J., Coord. Chem. Rev., 2002, vol. 232, nos. 1–2, p. 69. https://doi.org/10.1016/S0010-8545(02)00312-0
Vacca, A., Bruno, M., Boccarelli, A., Coluccia, M., Ribatti, D., Bergamo, A., Garbisa, S., Sartor, L., and Sava, G., British J. Cancer, 2002, vol. 86, no. 6, p. 993. https://doi.org/10.1038/sj.bjc.6600176
Sava, G., Zorzet, S., Turrin, C., Vita, F., Soranzo, M., Zabucchi, G., Cocchietto, M., Bergamo, A., DiGiovine, S., Pezzoni, G., Sartor, L., and Garbisa, S., Clin. Cancer Res., 2003, vol. 9, p. 1898. https://pubmed.ncbi.nlm.nih.gov/12738748/
Groessl, M., Reisner, E., Hartinger, C.G., Eichinger, R., Semenova, O., Timerbaev, A.R., Jakupec, M.A., Arion, V.B., and Keppler, B.K., J. Med. Chem., 2007, vol. 50, no. 9, p. 2185. https://doi.org/10.1021/jm061081y
Kapitza, S., Jakupec, M. A., Uhl, M., Keppler, B.K., and Marian, B., Cancer Lett., 2005, vol. 226, no. 2, p. 115 . https://doi.org/10.1016/j.canlet.2005.01.002
Hartinger, C.G., Metzler-Nolte, N., and Dyson, P.J., Organometallics, 2012, vol. 31, p. 5677.
Wang, F., Habtemariam, A., Erwin P. L. van der Geer, E.P.L., and Sadler, P.J., Proc. Natl. Acad. Sci. USA, 2005, vol. 102, no. 51, p. 18269. https://doi.org/10.1073/pnas.0505798102
Nowak-Sliwinska, P., van Beijnum, J.R., Casini, A., Nazarov, A.A., Wagnieres, G., van den Bergh, H., Dyson, P.J., and Griffioen, A.W., J. Med. Chem., 2011, vol. 54, no. 11, p. 3895. https://doi.org/10.1021/jm2002074
Meng, X., Leyva, M.L., Jenny, M., Gross, I., Benosman, S., Fricker, B., Harlepp, S., Hébraud, P., Boos, A., Wlosik, P., Bischoff, P., Sirlin, C., Pfeffer, M., Loeffler, J.-P., and Gaiddon, C., Cancer Res., 2009, vol. 69, no. 13, p. 5458. https://doi.org/10.1158/0008-5472.CAN-08-4408
Wai-Lun, Kwong., Kar-Yee, Lam., Chun-Nam, Lok., Yau-Tsz, Lai., Pui-Yan, Lee., and Chi-Ming, Che., Angew. Chem., 2016, vol. 55, no. 43, p. 13524. https://doi.org/10.1002/anie.201608094
Guo, W., Zheng, W., Luo, Q., Li, X., Zhao, Y., Xiong, S., and Wang, F., Inorg. Chem., 2013, vol. 52, no. 9, p. 5328. https://doi.org/10.1021/ic4002626
Caruso, F., Rossi, M., Benson, A., Opazo, C., Freedman, D., Monti, E., Gariboldi, M.B., Shaulky, J., Marchetti, F., Pettinari, R., and Pettinari, C., J. Med. Chem., 2012, vol. 55, no. 3, p. 1072. https://doi.org/10.1021/jm200912j
Maheshwari, R.K., Singh, A.K., Gaddipati, J., and Srimal, R.C., Life Sci., 2006, vol. 78, no. 18, p. 2081. https://doi.org/10.1016/j.lfs.2005.12.007
Kandioller, W., Balsano, E., Meier, S.M., Jungwirth, U., Göschl, S., Roller, A., Jakupec, M. A., Berger, W., Keppler, B.K., and Hartinger, C.G., Chem. Commun., 2013, vol. 49, p. 3348. https://doi.org/10.1039/C3CC40432C
Lim, H. and Draelos, Z., Informa Healthcare USA, New York, 2009.
Gupta, G., Cherukommu, S., Srinivas, G., Lee, S.W., Hwan, S., Mun, J., Jung, N.N., and Lee, C.Y., Lett. J. Inorg. Biochem., 2018, vol. 189, p. 17, no. 12. https://doi.org/10.1016/j.jinorgbio.2018.08.009
Nitiss, J.L., Nat. Rev. Cancer., 2009, vol. 9, no. 5, p. 338. https://doi.org/10.1038/nrc2607
Riccardo, P., Fabio, M., Agnese, P., Claudio, P., Giulio, L., Piotr, S., Rosario, S., Tina, R., and Dyson, P.J., Organometallics, 2016, vol. 35, no. 21, p. 3734. https://doi.org/10.1021/acs.organomet.6b00694
Dwyer, F.P., Gyarfas, E.C., Rogers, W.P., and Koah, J.H., Nature, 1952, vol. 170, no. 8318, p. 190. https://doi.org/10.1038/170190a0
Nagamani, C., Reddy, P.V., Reddy, M.R., Reddy, K.L., and Satyanarayana, S., Russ. J. Gen. Chem., 2020, vol. 90, no. 12, p. 2456. https://doi.org/10.1134/S1070363220120385
Shridhar, P., Purushothaman, S., and Ganeshpandian, M., Russ. J. Gen. Chem., 2020, vol. 90, no. 11, p. 2170. https://doi.org/10.1134/S1070363220110249
Yanyan, M., Weber, D.K., Buck, D.P., Motti, C.A., Collins, J.G., and Keene, E.R., Dalton Trans., 2011, vol. 40, no.7, p.1510. https://doi.org/10.1039/C0DT01250E
Li, F., Collins, J.G., and Keene, F.R., Chem. Soc. Rev., 2015, vol. 44, p. 2529. https://doi.org/10.1039/C4CS00343H
Mason, A.J., Marquette, A., and Bechinger, B., Biophys. J., 2007, vol. 93, no. 12, p. 4289. https://doi.org/10.1529/biophysj.107.116681
Petr, B., Vojtech, A., Ladislav, H., and Rene, K., Curr. Pharm. Anal., 2009, vol. 5, p. 47. https://doi.org/10.2174/157341209787314936
Rondevaldova, J., Novy, P., and Kokoska, L., Phytother. Res., 2014, vol. 29, p. 144. https://doi.org/10.1002/ptr.5237
Didier, B., Don Antoine, L., and Elisabeth, D.-C., Curr. Pharm. Des., 2013, vol. 19, no. 14, p. 2512. https://doi.org/10.2174/1381612811319140003
Crosby, I.T., Bourke, D.G., Jones, E.D., de Bruyn, P.J., Rhodes, D., Vandegraaff, N., Cox, S., Coates, J.A.V., and Robertson, A.D., Bioorg. Med. Chem., 2010, vol. 18, p. 6442. https://doi.org/10.1039/C7RA00825B
Wellington, K.W., RSC Adv., 2015, vol. 5, p. 20309. https://doi.org/10.1039/C4RA13547D
Ong, J.Y.H., Yong, P.V.C., Lim, Y.M., and Ho, A.S.H., Life Sci., 2015, vol. 135, no. 8, p. 158. https://doi.org/10.1016/j.lfs.2015.03.019
Brenner, A., Reikvam, H., Lavecchia, A., and Bruserud, Ø., Molecules, 2014, vol. 19, no. 19, no. 11, p. 18414. https://doi.org/10.3390/molecules191118414
Mario, K., Wolfgang, K., Kunwoo, K., Robert, F., Anderson, Erik, K., Michael, A.J., Alexander, R., TiloSöhnel, B.K.K., and Christian, G.H., Dalton Trans., 2016, vol. 45, p. 13091.
Sava, G., Bergamo, A., and Dyson, P.J., Dalton Trans., 2011, vol. 40, p. 9069.
Reedijk, J., Platin. Met. Rev., 2008, vol. 52, no. 1, p. 2. https://doi.org/10.1595/147106708X255987
Scolaro, C., Chaplin, A.B., Hartinger, C.G., Bergamo, A., Cocchietto, M., Keppler, B.K., Sava, G., and Dyson, P.J., Dalton Trans., 2007, vol. 43, p. 5065.
Dorcier, A., Dyson, P.J., Gossens, C., Rothlisberger, U., Scopelliti, R., and Tavernelli, I., Organometallics, 2005, vol. 24, p. 2114. https://doi.org/10.1021/om049022a
Ang, W.H.A., De Luca, C., Chapuis-Bernasconi, L., Juillerat-Jeanneret, M., Lo, B., and Dyson, P.J., Chem. Med. Chem., 2007, vol. 46, no. 19, p. 1799.
Huxham, L.A., Cheu, E.L.S., Patrick, B.O., and James, B.R., Inorg. Chim. Acta, 2003, vol. 352, no. 8, p. 238. https://doi.org/10.1016/S0020-1693(03)00155-5
Morris, R.E., Aird, R.E., Murdoch, P.D.S., Chen, H., Cummings, J., Hughes, N.D., Parsons, S., Parkin, A., Boyd, G., Jodrell, D.I., and Sadler, P.J., J. Med. Chem., 2001, vol. 44, p. 3616. https://doi.org/10.1021/jm010051m
Mendoza-Ferri, M.G., Hartinger, C.G., Eichinger, R.E., Stolyarova, N., Severin, K., Jakupec, M.A., Nazarov, A.A., and Keppler, B.K., Organometallics, 2008, vol. 27, no. 11, p. 2405. https://doi.org/10.1021/om800207t
Adams, R.D., Captain, B., and Trufan, E.J., Cluster Sci., 2007, vol. 18, no. 3, p.742. https://doi.org/10.1007/s10876-007-0133-x
Allardyce, C.S. and Dyson, P.J., J. Cluster Sci., 2001, vol. 12, no. 4, p. 563. https://doi.org/10.1023/A:1014294231261
Gupta, R.K., Pandey, R., Sharma, G., Prasad, R., Koch, B., Srikrishna, S., Li, P.Z., Xu, Q., and Pandey, D.S., Inorg. Chem., 2013, vol. 52, no. 7, p. 3687. https://doi.org/10.1021/ic302196v
Gupta, G., Garci, A., Murray, B.S., Dyson, P.J., Fabre, G., Trouillas, P., Giannini, F., Furrer, J., Suss-Fink, G., and Therrien, B., Dalton Trans., vol. 42, no. 43, p. 15457. https://doi.org/10.1039/C3DT51991K
Monfared, H.H., Vahedpour, M., Yeganeh, M.M., Ghorbanloo, M., Mayer, P., and Janiak, C., Dalton Trans., 2011, vol. 40, no. 6, p. 1286. https://doi.org/10.1039/C0DT00371A
Patai, S., The Chemistry of Carbon–Nitrogen Double Bond, New York: Interscience, 1970.
Nanjan, M., Subramanian, M., and Rengan, R., J. Organomet. Chem., 2016, vol. 807, no. 4, p. 45. https://doi.org/10.1016/j.jorganchem.2016.01.033
Gaiddon, C., Jeannequin, P., Bischoff, P., Pfeffer, M., Sirlin, C., and Loeffler, J.P., J. Pharmacol. Exp. Ther., 2005, vol. 315, no. 3, p. 1403. https://doi.org/10.1124/jpet.105.089342
Huang, H., Zhang, P., Yu, B., Chen, Y., Wang, J., Ji, L., and Chao, H., J. Med. Chem., 2014, vol. 57, no. 21, p. 8971. https://doi.org/10.1021/jm501095r
Ahmad, I.M., Aykin-Burns, N., Sim, J.E., Walsh, S.A., Higashikubo, R., Buettner, G.R., Venkataraman, S., Mackey, M.A., Flanagan, S.W., Oberley, L.W., and Spitz, D.R., J. Biol. Chem., 2005, vol. 280, no. 6, p. 4254. https://doi.org/10.1074/jbc.M411662200
Takahiro, M., Hiroshi, S., Misaki, N., and Yasuo, N., Chem. Pharm. Bull., 2016, vol. 64, no. 3, p. 282. https://www.jstage.jst.go.jp/article/cpb/64/3/64_c15-00903/_pdf
Graminha, A.E., Rodrigues, C., Batista, A.A., Teixeira, L.R., Fagundes, E.S., and Beraldo, H., Spectrochim. Acta, Part A, 2008, vol. 69, no. 4, p. 1073. https://doi.org/10.1016/j.saa.2007.06.005
Heinrich, T.A., Von Poelhsitz, G., Reis, R.I., Castellano, E.E., Neves, A., Lanznaster, M., Machado, S.P., Batista, A.A., and Costa-Neto, C.M., Eur. J. Med. Chem., 2011, vol. 46, no. 9, p. 3616. https://doi.org/10.1016/j.ejmech.2011.04.064
Lima, A.P., Pereira, F.C., Almeida, M.A.P., Mello, F.M.S., Pires, W.C., Pinto, T.M., Delella, F.K., Felisbino, S.L., Moreno, V., Batista, A.A., and Silveira-Lacerda, E.P., Plos One, vol. 9, no. 10., p. e105865 https://doi.org/10.1371/journal.pone.0105865
Adam, N.B., Lionel, M., and Jacqueline, K.B., J. Am. Chem. Soc., 2016, vol. 138, no. 15, p. 5020. https://doi.org/10.1021/jacs.6b02022
Friedman, A.E., Chambron, J.C., Sauvage, J.P., Turro, N.J., and Barton, J.K., J. Am. Chem. Soc., 1990, vol. 112, p. 4960.
Olson, E.J.C., Hu, D., Hormann, A., Jonkman, A.M., Arkin, M.R., Stemp, E.D.A., Barton, J.K., and Barbara, P.F., J. Am. Chem. Soc., 1997, vol. 119, no. 47, p. 11458. https://doi.org/10.1021/ja971151d
Gill, M.R. and Thomas, J.A., Chem. Soc. Rev., 2012, vol. 41, p. 3179. https://doi.org/10.1039/C2CS15299A
Baggaley, E., Weinstein, J.A., and Williams, J.A., Coord. Chem. Rev., 2012, vol. 256, nos. 15–16, p. 1762. https://doi.org/10.1016/j.ccr.2012.03.018
Knoll, J.D. and Turro, C., Coord. Chem. Rev., 2015, vol. 282, no. 1, p. 110. https://doi.org/10.1016/j.ccr.2014.05.018
Lim, M.H., Song, H., Olmon, E.D., Dervan, E.E., and Barton, J.K., Inorg. Chem., 2009, vol. 48, no. 12, p. 5392. https://doi.org/10.1021/ic900407n
Song, H., Kaiser, J.T., and Barton, J.K., Nat. Chem., 2012, vol. 4, no.8, p. 615. https://doi.org/10.1038/nchem.1375
McConnell, A.J., Lim, M.H., Olmon, E.D., Song, H., Dervan, E.E., and Barton, J.K., Inorg. Chem., 2012, vol. 51, no. 22, p. 12511. https://doi.org/10.1021/ic3019524
Prier, C.K., Rankic, D.A., and MacMillan, D.W.C., Chem. Rev., 2013, vol. 113, no. 7, p. 5322. https://doi.org/10.1021/cr300503r
Narayanam, J.M.R. and Stephenson, C.R.J., Chem. Soc. Rev., 2011, vol. 40, no. 1, p. 102. https://doi.org/10.1039/B913880N
Lin, S., Ischay, M.A., Fry, C.G., and Yoon, T.P., J. Am. Chem. Soc., 2011, vol. 133, no. 48, p. 19350. https://doi.org/10.1021/ja2093579
Schnermann, M.J. and Overman, L.E., Angew. Chem. Int. Ed., 2012, vol. 51, no. 38, p. 9576. https://doi.org/10.1002/anie.201204977
Zhao, J., Wu, W., Sun, J., and Guo, S., Chem. Soc. Rev., 2013, vol. 42, p. 5323.
Gatto, M.T., Firuzi, O., Agostino, R., Grippa, E., Borsò, A., Spinelli, F., Pavan, L., Petrolati, M., Petrucci, R., Marrosu, G., and Saso, L., Biomed. Chromatogr., 2002, vol. 16, no. 6, p. 404. https://doi.org/10.1002/bmc.174
Murata, M., Ivandini, T.A., Shibata, M., Nomura, S., Fujishim, A., and Einaga, Y., J. Electroanal. Chem., 2008, vol. 612, no. 1, p. 29. https://doi.org/10.1016/j.jelechem.2007.09.006
Weiss, S.J., Klein, R., Slivka, A., and Wei, M., J. Clin. Invest., 1982, vol. 70, no. 3, p. 598. https://doi.org/10.1172/jci110652
Kowada, T., Maeda, H., and Kikuchi, K., Chem. Soc. Rev., 2015, vol. 44, p. 4953.
Yuan, L., Lin, W., Zheng, K., He, L., and Huang, W., Chem. Soc. Rev., 2013, vol. 42, p. 622.
Balzani, V., Bergamini, G., Marchioni, F., and Ceroni, P., Coord. Chem. Rev., 2006, vol. 250, nos. 11–12, p. 1254. https://doi.org/10.1016/j.ccr.2005.11.013
Zeng, L., Gupta, P., Chen, Y., Wang, E., Ji, L., Chao, H., and Chen, Z.-S., Chem. Soc. Rev., 2017, vol. 46, no. 19, p. 5771. https://doi.org/10.1039/c7cs00195a
Milek, M., Heinemann, F.W., and Khusniyarov, M.M., Inorg. Chem., 2013, vol. 52, no. 19, p. 11585. https://doi.org/10.1021/ic401960x
Liu, F., Gao, Y., Wang, J., and Sun, S., Analyst, 2014, vol. 139, p. 3324.
Cao, L., Zhang, R., Zhang, W., Du, Z., Liu, C., Ye, Z., Song, B., and Yuan, J., Biomaterials, 2015, vol. 68, p. 21. https://doi.org/10.1016/j.biomaterials.2015.07.052
Pierre, V.C., Kaiser, J.T., and Barton, J.K., Proc. Natl. Acad. Sci. USA, 2007, vol. 104, no. 2, p. 429. https://doi.org/10.1073/pnas.0610170104
Chatterjee, D.K., Fong, L.S., and Zhang, Y., Adv. Drug. Delivery Rev., 2008, vol. 60, no. 15, p. 1627. https://doi.org/10.1016/j.addr.2008.08.003
Schweitzer, C. and Schmidt, R., Chem. Rev., 2003, vol. 103, no. 5, p. 1685. https://doi.org/10.1021/cr010371d
Mendoza-Ferri, M.G., Hartinger, C.G., Mendoza, M.A., Groessl, M., Egger, A. E., Eichinger, R.E., Mangrum, J.B., Farrell, N.P. Maruszak, M., Bednarski, P.J., Klein, F., Jakupec, M.A., Nazarov, A.A., Severin, K., and Keppler, B.K., J. Med. Chem., 2009, vol. 52, no. 4, p. 916. https://doi.org/10.1021/jm8013234
Davia, K., King, D., Hong, Y., and Swavey, S., Inorg. Chem. Commun., 2008, vol. 11, no. 5, p. 584. https://doi.org/10.1016/j.inoche.2008.02.023
Liu, Y., Hammitt, R., Lutterman, D.A., Joyce, L.E., Thummel, R.P., and Turro, C., Inorg. Chem., 2009, vol. 48, no. 1, p. 375. https://doi.org/10.1021/ic801636u
Erkkila, K.E., Odom, D.T., and Barton, J.K., Chem. Rev., 1999, vol. 99, no. 9, p. 2777. https://doi.org/10.1021/cr9804341
Zeglis, B.M., Boland, J.A., and Barton, J.K., J. Am. Chem. Soc., 2008, vol. 130, no. 24, p. 7530. https://doi.org/10.1021/ja801479y
Bergamo, A. and Sava, G., Dalton Trans., 2007, p. 1267
Ayman, A., Abdel-Shafi, P., Beer, P.D., Mortimer, R.J., and Wilkinson, F., PhysChemChemPhys, 2000, vol. 2, no. 14, p. 3137.
Juris, A., Balzani, V., Barigelletti, F., Campagna, S., Belser, P., and Von Zelewsky, A., Coord. Chem. Rev., 1988, vol. 84, p. 85. https://doi.org/10.1016/0010-8545(88)80032-8
Coe, B., J. Acc. Chem. Res., 2006, vol. 39, no. 6, p. 383. https://doi.org/10.1021/ar050225k
Samoc, M., Morrall, J.P., Dalton, G.T., Cifuentes, M.P., and Humphrey, M.G., Angew. Chem. Int. Ed., 2007, vol. 46, no. 5, p. 731. https://doi.org/10.1002/anie.200602341
Girardot, C., Lemercier, G., Mulatier, J.-C., Chauvin, J., Baldeck, P.L., and Andraud, C., Dalton Trans., 2007, p. 3421. https://doi.org/10.1039/B706715A
Mark, P., Molloy, M., and McDowell, T., Separat. Sci. Technol., 2005, vol. 7, p. 123. https://doi.org/10.1016/S0149-6395(05)80009-1
Shintani, R., Isobe, S., Takeda, M., and Hayashi, T., Angew. Chem. Int. Ed., 2010, vol. 49, no. 22, p. 3795. https://doi.org/10.1002/anie.201000937
Wu, P., Lai-Ming, W.E., Ma, D.L., Glenna, S.-M.T., Ng, K.-M., and Che, C.-M., Chem. Eur. J., 2009, vol. 15 no. 15, p. 3623. https://doi.org/10.1002/chem.200990048
Sauvage, J.P., Collin, J.P., Chambron, J.C., Guillerez, S., Coudret, C., Balzani, V., Barigelletti, F., Cola, L.D., and Flamigni, L., Chem. Rev., 1994, vol. 94, no. 4, p. 993. https://doi.org/10.1021/cr00028a006
Chun-Yuen, Wong., Lai-Hon, Chung., Sheng, Lin., Daniel Shiu-Hin, Chan., Chung-Hang, Leung., and Dik-Lung, Ma., Sci. Rep., 2014, vol. 4, p. 7136.
Roberson, E.D. and Mucke, L., Science, 2006, vol. 314, no. 5800, p. 781. https://doi.org/10.1126/science.1132813
Skaper, S.D., Int. Rev. Neurobiol., 2012, vol. 102, p. 277. https://doi.org/10.1016/B978-0-12-386986-9.00011-9
Hardy, J. and Selkoe, D.J., Science, 2002, vol. 297, no. 5580, p. 353. https://doi.org/10.1126/science.1072994
Ranade, D.S., Bapat, A.M., Ramteke, S.N., Joshi, B.N., Roussel, P., Tomas, A.P.D., and Kulkarni, P.P., Eur. J. Med. Chem., 2015. https://doi.org/10.1016/j.ejmech.2015.07.028
Guisasola, E.E.B., Andujar, S.A., Hubin, E., Broersen, K., Kraan, I.M., Méndez, L., Delpiccolo, C.M.L., Masman, M.F., Rodríguez, A.M., and Enri, R.D., Eur. J. Med. Chem., 2015, vol. 95, p.136. https://doi.org/10.1016/j.ejmech.2015.03.042
Tarus, B., Nguyen, P.H., Berthoumieu, O., Faller, P., Doig, A.J., and Derreumaux, P., Eur. J. Med. Chem., 2015, vol. 91, p. 43. https://doi.org/10.1016/j.ejmech.2014.07.002
Guzior, N., Bajda, M., Skrok, M., Kurpiewska, K., Lewiński, K., Brus, B., Pišlar, A., Kos, J., Gobec, S., and Malawska, B., Eur. J. Med. Chem., 2015, vol. 92, p. 738. https://doi.org/10.1016/j.ejmech.2015.01.027
Kenche, V.B., Hung, L.W., Perez, K., Volitakes, I., Ciccotosto, G., Kwok, J., Critch, N., Sherratt, N., Cortes, M., Lal, V., Masters, C.L., Murakami, K., Cappai, R., Adlard, P.A., and Barnham, K.J., Angew. Chem. Int. Ed. Engl., 2013, vol. 52, no. 12, p. 3374. https://doi.org/10.1002/anie.201209885
Sasaki, I., Bijani, C., Ladeira, S., Bourdon, V., Faller, P., and Hureau, C., Dalton Trans., 2012, vol. 41, p. 6407. https://doi.org/10.1039/c2dt12177h
Wang, X., Wang, X., Zhang, C., Jiao, Y., and Guo, Z., Chem. Sci., 2012, vol. 3, no. 4, p. 1304. https://doi.org/10.1039/C2SC01100J
Yellol, G.S., Yellol, J.G., Kenche, V.B., Liu, X.M., Barnham, K.J., Donaire, A., Janiak, C., and Ruiz, J., Inorg. Chem., 2015, vol. 54, no. 2, p. 470. https://doi.org/10.1021/ic502119b
Man, Y.-W.B., Chan, H.-M., Leung, C.-H., Chan, D.S.-H., Bai, L.-P., Jiang, Z.-H., Li, H.-W., and Ma, D.-L., Chem. Sci., 2011, vol. 2, no. 5, p. 917. https://doi.org/10.1039/C0SC00636J
Kumar, A., Moody, L., Olaivar, J.F., Lewis, N.A., Khade, R.L., Holder, A.A., Zhang, Y., and Rangachari, V., ACS Chem. Neurosci., 2010, vol. 1, no. 10, p. 691. https://doi.org/10.1021/cn100046m
Yu, H., Li, M., Liu, G., Geng, J., Wang, J., Ren, J., Zhaoa, C., and Qu, X., Chem. Sci., 2012, vol. 3, no. 11, p. 3145. https://doi.org/10.1039/C2SC20372C
Messori, L., Camarri, M., Ferraro, T., Gabbiani, C., and Franceschini, D., ACS Med. Chem. Lett., 2013, vol. 4, no. 3, p. 329. https://doi.org/10.1021/ml3003567
Valensin, D., Gabbiani, C., and Messori, L., Coord. Chem. Rev., 2012, vol. 256, nos. 19–20, p. 2357. https://doi.org/10.1016/j.ccr.2012.04.010
Delangle, P. and Mintz, E., Dalton Trans., 2012, vol. 41, no. 21, p. 6359. https://doi.org/10.1039/C2DT12188C
Suarez-Almazor, M.E., Belseck, E., Homik, J., Dorgan, M., and Ramos-Remus, C., The Cochrane Library, Control Clin. Trials, 2000, vol. 21, no. 5, p. 476. https://doi.org/10.1016/s0197-2456(00)00067-2
Clements, P.J., Hurwitz, E.L., W.K, Wong, Seibold, J.R., Mayes, M., White, B., Wigley, F., Weisman, M., Barr, W., Moreland, L., Medsger, Jr.T.A., Steen, V.D., Martin, R.W., Collier, D., Weinstein, A., Lally, E., Varga, J., Weiner, S.R., Andrews, B., Abeles, M., and Furst, D.E., Arthritis Rheum., 2000, vol. 43, no. 11, p. 2445. https://doi.org/10.1002/1529-0131(200011)43:11%3C2445::AID-ANR11%3E3.0.CO;2-Q
Vora, A., Mitchell, C.D., Lennard, L., Eden, T.O., Kinsey, S.E., Lilleyman, J., and Richards, S.M., Lancet, 2006, vol. 368, no. 9544, p. 1339. https://doi.org/10.1016/s0140-6736(06)69558-5
WHO Model List of Essential Medicines. www.who.int/ medicines/publications/essentialmedicines/en
Song, L., Guo, Z., and Chen, Y., Electrophoresis, 2012, vol. 33, no. 14, p. 2056. https://doi.org/10.1002/elps.201200169
Neuroscience, Purves, D., Augustine, G.J., Fitzpatrick, D., Hall, W.C., LaMantia, A.S., McNamara, J.O., and White L.E., Eds., Sinauer Associates, Sunderland, 2008, p. 137.
Mazloum-Ardakani, M. and Khoshroo, A., Electrochem. Commun., 2014, vol. 42, p. 9. https://doi.org/10.1016/j.elecom.2014.01.026
Tiwari, I., Gupta, M., Sinha, P., and Aggarwal, S.K., Electrochimica Acta, 2012, vol. 76, p. 106. https://doi.org/10.1016/j.electacta.2012.04.053
Mazloum-Ardakani, M., Ali, S.-M., and Masoud, S.-N., Electroanalysis, 2016, vol. 28, no. 6, p.1370. https://doi.org/10.1002/elan.201500597
Li, B. and Dixneuf, P.H., Chem. Soc. Rev., 2013, vol. 42, no. 13, p. 5744. https://doi.org/10.1039/C3CS60020C
Arockiam, P. B., Bruneau, C., and Dixneuf, P.H., Chem. Rev., 2012, vol. 112, p. 5879. https://doi.org/10.1021/cr300153j
Seki, M., Org. Process Res. Dev., 2016, vol. 20, no. 5, p. 867. https://doi.org/10.1021/acs.oprd.6b00116
Diers, E., Kumar, N.Y.P., Mejuch, T., Marek, I., and Ackermann, L., Tetrahedron, 2013, vol. 69, no. 22, p. 4445. https://doi.org/10.1016/j.tet.2013.01.006
Zell, D., Warratz, S., Gelman, D., Garden, S.J., and Ackermann, L., Chem. Eur. J., 2016, vol. 22, no. 4, p. 1248. https://doi.org/10.1002/chem.201504851
Simonetti, M., Perry, G.J.P., Cambeiro, X.C., Juliá-Hernández, F., Arokianathar, J.N., and Larrosa, I.J., Am. Chem. Soc., 2016, vol. 138, no. 10, p. 3596. https://doi.org/10.1021/jacs.6b01615
Seki, M., Synthesis, 2015, vol. 47, no. 19, p. 1423. https://doi.org/10.1055/s-0034-1378848
Wang, D.-H., Engle, K.M., Shi, B.-F., and Yu, J.-Q., Science, 2010, vol. 327, no. 5963, p. 315. https://www.jstor.org/stable/40508531
Davies, H.M.L. and Manning, J.R., Nature, 2008, vol. 451, no. 7177, p. 417. https://doi.org/10.1038/nature06485
Li, J., Warratz, S., Zell, D., Sarkar, S.D., Ishikawa, E.E., and Ackermann, L.J., Am. Chem. Soc., 2015, vol. 137, no. 43, p. 13894. https://doi.org/10.1021/jacs.5b08435
Jonathan, H. and Lutz, A., Eur. J. Org. Chem., vol. 2016, no. 22, p. 3700. https://doi.org/10.1002/ejoc.201600742
Desilvestro, J., Grätzel, M., Kavan, L., Moser, J., and Augustynski, J., J. Am. Chem. Soc., 1985, vol. 107, p. 2988. https://doi.org/10.1021/ja00296a035
Nazeeruddin, M. K., Péchy, P., Renouard, T., Zakeeruddin, S.M., Humphry-Baker, R., Comte, P., Liska, P., Cevey, L., Costa, E., Shklover, V., Spiccia, L., Deacon, G.B., Bignozzi, C.A., and Grätzel, M., J. Am. Chem. Soc., 2001, vol. 123, no. 8, p. 1613. https://doi.org/10.1021/ja003299u
Asghar, M.I., Miettunen, K., Halme, J., Vahermaa, P., Toivola, M., Aitola, K., and Lund, P., Energy Environ. Sci., 2010, vol. 3, p. 418. https://doi.org/10.1039/B922801B
Chiba, Y., Islam, A., Watanabe, Y., Komiya, R., Koide, N., and Han, L., Jpn. J. Appl. Phys., 2006, vol. 45, p. L638.
Nusbaumer, H. Zakeeruddin, S.M., Moser, J.E., and Grätzel, M., Chem.-Eur. J., 2003, vol. 9, no. 16, p. 3756. https://doi.org/10.1002/chem.200204577
Ohta, M., Koumura, N., Hara, K., and Mori, S., Electrochem. Commun., 2011, vol. 13, no. 9, p. 778. https://doi.org/10.1016/j.elecom.2011.06.022
Feldt, S.M., Gibson, E.A., Gabrielsson, E., Sun, L., Boschloo, G., and Hagfeldt, A., J. Am. Chem. Soc., 2010, vol. 132, no. 46, p. 16714. https://doi.org/10.1021/ja1088869
Mathew, S., Yella, A., Gao, P., Humphry-Baker, R., Curchod, B.F.E., Ashari-Astani, N., Tavernelli, I., Rothlisberger, U., Nazeeruddin, M.K., and Graetzel, M., Nat. Chem., 2014, vol. 6, no. 3, p. 242. https://doi.org/10.1038/nchem.1861
Polander, L.E. Yella, A., Curchod, B.F.E., Ashari Astani, N., Teuscher, J., Scopelliti, R., Gao, P., Mathew, S., Moser, J.-E., Tavernelli, I., Rothlisberger, U., Graetzel, M., Nazeeruddin, M.K., and Frey, J., Angew. Chem., Int. Ed., 2013, vol. 52, no. 33, p. 8731. https://doi.org/10.1002/anie.201304608
Wu, K.-L., Clifford, J.N., Wang, S.-W., Aswani, Y., Palomares, E., Lobello, M. G., Mosconi, E., De Angelis, F., Ku, W.-P., Chi, Y., Nazeeruddin, M.K., and Graetzel, M., ChemSusChem., 2014, vol. 7, no. 10, p. 2930. https://doi.org/10.1002/cssc.201402030
Yao, Z., Zhang, M., Wu, H., Yang, L., Li, R., and Wang, P., J. Am. Chem. Soc., 2015, vol. 137, no. 36, p. 3799. https://doi.org/10.1021/jacs.5b07394
Mosconi, E., Yum, J.-H., Kessler, F., Gomez Garcia, C.J., Zuccaccia, C., Cinti, A., Nazeeruddin, M.K., Graetzel, M., and De Angelis, F., J. Am. Chem. Soc., 2012, vol. 134, no. 47, p. 19438. https://doi.org/10.1021/ja3079016
Wu, K.-L., Li, C.-H., Chi, Y., Clifford, J.N., Cabau, L., Palomares, E., Cheng, Y.-M., Pan, H.-A., and Chou, P.-T., J. Am. Chem. Soc. 2012, vol. 134, no. 17, p. 7488. https://doi.org/10.1021/ja300828f
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Guin, P.S., Roy, S. Recently Reported Ru-Metal Organic Coordination Complexes and Their Application (A Review). Russ J Gen Chem 92, 1546–1561 (2022). https://doi.org/10.1134/S1070363222080242
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222080242