Abstract
Coordination nature in the structures between the heterocyclic sulfonic acid group and heavy metal atoms, structural optimization, their physical and chemical properties have been studied. Herein, a new compound [Hg2(P–SO3)(NO3)]n (1) has been synthesized via solvent volatilization method, according to which pyrazine sulfonic acid (P–SO3H) has been used as the major ligand, and nitrate has been used as the precursor. X-Ray single-crystal diffraction analysis demonstrates that 1 belongs to the Pca21 space group. Both pyrazine sulfonate and nitrate serve as chelate bridging linkers for the dimercury(I) ions (Hg22+or HgI–HgI) forming the 2D layer coordination polymer involved in further construction of the stable 3D network through the π–π stacking of pyrazine rings. Thermal stability and fluorescence properties of 1 have been closely studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Nowadays, design, synthesis and investigation of the coordination polymers (CPs) attract considerable attention due to the progress of coordination chemistry and crystal engineering because of their diverse topological structures [1–3] and potential applications in such areas as magnetic materials [4–6], biomedicine [7], catalysis [8, 9], luminescence [10–12], chemical sensing [13–15], and more.
Some researchers hypothesized that possible coordination of mercury ions with ligands could form stable coordination polymers that reduce toxicity of mercury [16,17]. For instance, Suresh et al. [18] reported that a dual detection probe developed from an adenine-based luminescent coordination polymer for the selective sensing of mercury ions in an aqueous phase, identified mercury ions in lethal environments. Recently, Huang et al. [19] prepared triazine-based porous organic polymers with high BET surface area to remove mercury ions from aqueous solutions due to the strong chelating effect of oxygen and nitrogen with mercury ions.
On the other hand, nitrogen-containing heterocyclic sulfonic acid coordination polymers attracted close attention due to their unique physicochemical properties [19, 20]. Nitrogen atoms can act as σ-electron donors or π-electron acceptors in the course of coordination process, and sulfonates tend to form intramolecular or intermolecular hydrogen bonds, that are beneficial in assembling extended high-dimensional structures [21]. In our previous research one nitrogen-containing heterocyclic 2-pyridine-sulfonic acid was selected for the synthesis of Cu-based 3D supramolecular polymer with the multiple interpenetration structure [22]. Lately, nitrogen-containing heterocyclic pyrazine sulfonic acid (P–SO3H) has been used in constructing two Ag and one Na–Cu CPs [23, 24]. However, lack of extensive investigations on the mercury compounds containing N-heterocyclic sulfonic acid limited their application unlike those of transition metal CPs. Therefore, we designed and synthesized a new dimercury(I)-based pyrazine sulfonate polymer [Hg2(P–SO3)(NO3)]nFootnote 1 (1) via the solvent evaporation method. The compound 1 has been characterized by single crystal X-ray diffraction, IR and fluorescence spectroscopy, and thermal analysis.
EXPERIMENTAL
All reagents were of analytical grade and used without additional purification. FT-IR spectra (KBr discs) were recorded on a PE Spectrum One spectrophotometer. Solid-state fluorescence spectra were recorded on a HORIBA JOBIN JVON fluorescence spectrophotometer, FL3-P-TCSPC, France. Thermogravimetric analysis was performed on a Labsys Evo-TGA, France.
The ligand was prepared in accordance with the developed earlier method [25]. Pyrazine sulfonic acid (P–SO3H, 0.3 mmol) and Hg(NO3)2·0.5H2O (0.5 mmol) were added to a mixture of ethanol and acetonitrile (2 : 3 ratio) of the certain volume. The mixture was placed in a dark container and stirred at 60°C for 5 h, then filtered, and the filtrate was volatilized at room temperature giving a yellow flake-like crystal after 8 days of storage, yield 12.5% (based on Hg). IR spectrum, ν, cm–1: 3434 (OH), 1632 (C=C), 1384 (C=N), 1237 (S=O), 1208 (S=O), 645 (C–S). Found, %: C 7.76; H 0.51; N 6.78. C3H4Hg2N3O6S. Calculated, %: C 7.72; H 0.49; N 6.75. M 622.
Diffraction data were collected on an Agilent Super-Nova diffractometer at 298 K (MoKα, λ = 0.71073 Å) and analyzed by Olex2 [26]. All the non-hydrogen atoms were refined by the SHELXTL program package using the full matrix least square method [27]. The accumulated crystallographic data, and the selected bond distances and angles determined for 1 are listed in Tables 1 and 2, respectively.
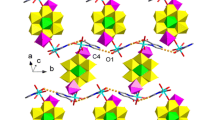
According to the single-crystal X-ray crystallography (Fig. 1a) Hg1 exhibited a distorted pentagonal geometry when coordinated with nitrogen and oxygen atoms of the deprotonated pyrazine sulfonic acid ligand (P–SO3). The bond distances Hg1–N1 [2.325(13) Å] and Hg1–O1 [2.54(3) Å] were shorter than those reported earlier [28]. The metal-metal bond was formed upon coordination of dimercury(I) ion (HgI–HgI), Hg2 and Hg1, and then linked by oxygen of the nitrate and two P–SO3 ligands. The bond distances Hg1–Hg2 [2.5246(16) Å], Hg2–O6 [2.63(3) Å] and Hg2–O3#1 [2.65(3) Å] were in agreement with those determined for the mercury polymers [29]. The structural unit could extend into the 2D network via sharing oxygen atoms of the P–SO3 ligand being coordinated with dimercury(I) ion. The contact angles of two sulfonate anions O6Hg2O3#1 and S1O3Hg2#3 were determined to be 136.4° and 134.5°, respectively (Figs. 1b, 1c). The 3D network could be constructed from the 2D structure via the weak π–π stacking of pyrazine rings (Fig. 1d).
According to TGA analysis weight loss of polymer 1 proceeded in three steps. In the range of room temperature to 218°C the first step of mass loss was due to gradual release of the guest nitrate linkers (Obs. 5.2%, Calcd 9.8%). Then, the rapid decomposition was recorded at ~358°C, and the maximum weight loss could be attributed to the loss of mercury pyrazine sulfonate species (Obs. 43.7%, Calcd 42.3%). Finally, the TGA curve decreased slowly upon further heating which led to pyrolysis and formation of mercury oxide at ~800°C (Obs. 35.8%, Calcd 34.9%).
To date, only limited data are available for luminescent properties of dimercury(I) based pyrazine sulfonate complexes. In order to fill in this gap, solid state luminescence was studied by scanning the polymer 1 and free P–SO3H at room temperature (Fig. 2).
The maximum emission signal for 1 was recorded at 448 nm (λex = 380 nm). The fluorescent intensity of polymer 1 was weaker than that of P–SO3H and demonstrated blue shift at 82 nm (λex = 461 nm, λem = 530 nm) which could be attributed to the heavy atom effect and electron transfer from the excited state (π orbital) of the ligand to the vacant 6s orbital of mercurous ion upon coordination [30]. Regarding the above, we presume that some of the ligand’s fluorescence energy might be transferred to the dimercury(I) ion (HgI–HgI) via the sulfonate moiety, resulting in the increase in the fluorescence quenching effect of 1. Nevertheless, the authenticity of this phenomenon should be scrutinized further.
CONCLUSIONS
A new dimercury(I) based pyrazine sulfonate polymer 1 has been synthesized using the solvent volatilization method. The single-crystal structure, thermal stability and fluorescence properties of it have been studied, and have indicated that the heterocyclic sulfonic acid ligand could acted as a bridging linker in coordination with different mercury(I) ions with different coordination environments, and form 3D network via π–π stacking.
AUTHOR CONTRIBUTIONS
Haojin Hu (undergraduate student) and Jinli Quan (undergraduate student) contributed equally to this work. Zhuojie Tan and Jun-Hong Fu participate in data analysis and results discussion; Dr. Yi-Jun Liang and Dr. Jun-xia Li: conceived the central idea, manuscript written, revised, and supervision. All authors have given approval to the final version of the manuscript.
Notes
Crystallographic data for the structural analysis have been deposited at the Cambridge Crystallographic Data Center with CCDC 1995512 for [Hg2(P–SO3)(NO3)]n (P–SO3=pyrazine sulfonic acid). Copies of this information may be obtained free of charge from the CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK (Fax: +44 1223 336 033; E-mail: deposit@ccdc.cam.ac.uk or www.ccdc.cam.ac.uk).
REFERENCES
Li, J.-X., Du, Z.-X., Zhang, L.-L., Liu, D.-L., and Pan, Q.-Y., Inorg. Chim. Acta, 2020, vol. 512, p. 119890. https://doi.org/10.1016/j.ica.2020.119890
Li, J.-X., Du, Z.-X., and Huang, W.-P., Synth. React. Inorg. Met.-Org. Chem., 2014, vol. 44, p. 352. https://doi.org/10.1080/15533174.2013.769588
Li, J.-X. and Du, Z.-X., Z. Naturforsch. B, 2015, vol. 70, p. 505. https://doi.org/10.1515/znb-2015-0010
Li, J.-X., Du, Z.-X., Xiong, L.-Y., Fu, L.-L., and Bo, W.-B., J. Solid State Chem., 2021, vol. 293, p.121799. https://doi.org/10.1016/j.jssc.2020.121799
Du, Z.-X. and Li, J.-X., Z. Naturforsch. B, 2020, vol. 75, nos. 6–7, p. 577. https://doi.org/10.1515/znb-2020-0042
Li, J.-X., Du, Z.-X., Pan, Q.-Y., Zhang, L.-L., and Liu, D.-L., Inorg. Chim. Acta., 2020, vol. 509, p. 119677. https://doi.org/10.1016/j.ica.2020.119677
Wang, D.-D., Jana, D.-L., and Zhao, Y.-L., Acc. Chem. Res., 2020, vol. 53, p. 1389. https://doi.org/10.1021/acs.accounts.0c00268
Bavykina, A., Kolobov, N., Khan, I.S., Bau, J.A., Ramirez, A., and Gascon, J., Chem. Rev., 2020, vol. 120, p. 8468. https://doi.org/10.1021/acs.chemrev.9b00685
Du, Z.-X. and Li, J.-X., Inorg. Chim. Acta., 2015, vol. 436, p. 159. https://doi.org/10.1016/j.ica.2015.07.036
Li, J.-X. and Du, Z.-X., J. Clust. Sci., 2020, vol. 31, p. 507. https://doi.org/10.1007/s10876-019-01666-w
Li, J.-X., Du, Z.-X., Wang, J., and Feng, X., Z. Naturforsch. B, 2019, vol. 74, nos. 11–12, p. 839. https://doi.org/10.1515/znb-2019-0147
Li, J.-X. and Du, Z.-X., J. Coord. Chem., 2016, vol. 69, p. 2563. https://doi.org/10.1080/00958972.2016.1216106
Yi, F.-Y., Chen, D., Wu, M.-K., Han, L., and Jiang, H.-L., ChemPlusChem., 2016, vol. 81, p. 675. https://doi.org/10.1002/cplu.201600137
Li, J.-X., Du, Z.-X., and Feng, X., Z. Naturforsch. B, 2019, vol. 74, nos. 11–12, p. 833. https://doi.org/10.1515/znb-2019-0128
Du, Z.-X., Li, J.-X., Liu, S.-J., Wang, Z.-Q., and Pan, Q.-J., Z. Naturforsch. B, 2020 , vol. 75, nos. 6–7, p. 567. https://doi.org/10.1515/znb-2020-0036
Pletz, J., Sánchez-Bayo, F., and Tennekes, H.-A., Toxicology, 2016, vol. 347, p. 1. https://doi.org/10.1016/j.tox.2016.02.006
Ding, S.-Y., Dong, M., Wang, Y.-W., Chen, Y.-T., Wang, H.-Z., Su, C.-Y., and Wang, W., J. Am. Chem. Soc., 2016, vol. 138, p. 3031. https://doi.org/10.1021/jacs.5b10754
Rachuri, Y., Parmar, B., Bisht, K.-K., and Suresh, E., Cryst. Growth Des., 2017, vol. 17, p. 1363. https://doi.org/10.1021/acs.cgd.6b01755
Peng, R.-X., Chen, G., Zhou, F., Man, R.-L., and Huang, J.-H., Chem. Eng. J., 2019, vol. 371, p. 260. https://doi.org/10.1016/j.cej.2019.04.063
Neelakandan, S., Ramachandran, R., Fang, M.L., and Wang, L., Int. J. Energ. Res., 2020, vol. 44, p. 1673. https://doi.org/10.1002/er.4981
Maity, D.-K., Otake, K., Ghosh, S., Kitagawa, H., and Ghoshal, D., Inorg. Chem., 2017, vol. 56, p.1581. https://doi.org/10.1021/acs.inorgchem.6b02674
Jiang, Y.M., Yin, Z., He, K.H., Zeng, M.H., and Kurmoo, M., Inorg. Chem., 2011, vol. 50, p. 2329. https://doi.org/10.1021/ic102020g
Liang, Y.-J., Feng, G., Zhang, X., Li, J.-X., and Jiang, Y., J. Struct. Chem., 2021, vol. 62, p. 300. https://doi.org/10.1134/S0022476621020153
Zheng, Z., Xu, P., Jiang, Y., Liang, Y.-J., and Li, J.-X., J. Struct. Chem. 2021, vol. 62, p. 292. https://doi.org/10.1134/S0022476621020141
Hort, E. and Spoerri, P.E., J. Am. Chem. Soc., 1948, vol. 70, p. 1657. https://doi.org/10.1021/ja01184a507
Dolomanov, O.-V., Bourhis, L.-J., Gildea, R.-J., Howard, J.A.K., and Puschmann, H., J. Appl. Crystallogr., 2009, vol. 42, p. 339. https://doi.org/10.1107/S0021889808042726
Sheldrick, G.M., SHELXS-97, Program for Crystal Structure Solution, Göttingen: Göttingen University, 1997.
Lo, H.-C., Chen, H., and Fish, R.-H., Eur. J. Inorg. Chem., 2001, vol. 2001, p. 2217. https://doi.org/10.1002/1099-0682(200109)2001:9<2217::AID-EJIC2217>3.0.CO;2-L
Brodersen, K., Comment Inorg. Chem., 1981, vol. 1, p. 207. https://doi.org/10.1080/02603598108078093
Li, F., Hong, Y.-S., Zuo, K.-X., Sun, Q., and Gao, E.-Q., J. Solid State Chem., 2019, vol. 270, p. 509. https://doi.org/10.1016/j.jssc.2018.12.025
Funding
The research was supported by the Key scientific research projects in Colleges and Universities of Henan province (no. 21A150036), the National Undergraduate Training Program for Innovation and Entrepreneurship (no. 20181184701) and the Scientific Research Program of High-Level Talents of Foshan University (no. Gg07118).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Hu, H., Quan, J., Tan, Z. et al. Synthesis and Properties of Dimercury(I) Crystal Network Constructed with Functionalized Pyrazine Sulfonate and Nitrate Linkers. Russ J Gen Chem 91, 910–914 (2021). https://doi.org/10.1134/S1070363221050224
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221050224