Abstract
Extraction of rare earths(III) from hydrochloric acid solutions with mixtures of new tridentate carbamoylmethylphosphine oxides Ph2P(O)CH2CON(R)CH2CH2P(O)Ph2 (R = Me, Bu, Oct) and 4-benzoyl-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one in organic solvents has been studied. The observed strong synergistic effect has been attributed to the formation of hydrophobic mixed-ligand rare earth complexes in the organic phase. Stoichiometry of the extractable complexes has been determined, and extraction constants have been calculated. The effect of organic solvent and composition of the aqueous phase on the extraction efficiency has been analyzed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Nowadays extraction technologies provide the main tool for the extraction and separation of rare earths, as well as for their separation from actinides, in the deep processing of nuclear wastes [1, 2]. Neutral polydentate organophosphorus compounds, especially carbamoylmethylphosphine oxides [3–8], exhibit a high extraction ability toward tervalent rare earths.
With the goal of enhancing the efficiency of extraction of rare earths from aqueous solution, the synergistic effect, i.e., non-additive increase of the partition coefficients of metal ions in their extraction with mixtures of acidic and neutral extractants in a low-polar solvent [9–11], has been extensively studied and utilized since the mid-20th century. Synergistic effect was revealed in the extraction of rare earths(III) with mixtures of chelating acidic extractants, such as β-diketones, 4-acylpyrazol-5-ones, 4-acyl-1,2-oxazol-5-ones, and picrolonic acid, with neutral donor extractants such as crown ethers [9], calixarenes [10, 12, 13], monodentate phosphoryl compounds [14–16], and bi- and polydentate carbamoylmethylphosphine oxides [15, 17–21]. According to the results of numerous studies, the synergistic effect originates from the formation in the organic phase of a mixed-ligand rare earth(III) complex which is more hydrophobic than the corresponding homoligand complexes [10, 16]. The formation of such complexes is favored by increase of the acidity of chelating reagents and complexing power of neutral extractants [10]. As a rule, the synergistic effect increases in parallel with the number of donor groups in the neutral extractant molecule [13, 20].
The present work was aimed at studying the effect of the structure of carbamoylmethylphosphine oxides on their extraction ability toward rare earths(III). For this purpose, we synthesized novel tridentate ({N-alkyl-N-[2-(diphenylphosphoryl)ethyl]carbamoyl}methyl)diphenylphosphine oxides 1–3 containing two phosphoryl groups [22] (Scheme 1). Study of the extraction of micro amounts of U(VI), Th(IV), and rare earths(III) with solutions of 1–3 showed that increase of the number of coordinating P=O groups significantly enhances their ability to extract tervalent rare earth ions from nitric acid solutions in comparison to structurally related bidentate carbamoylmethylphosphine oxides [23].
Herein, we report the results of studying extraction of rare earths(III) from chloride solutions with mixtures of tridentate phosphine oxides 1–3 [22] and 4-benzoyl-1-phenyl-3-methyl-1H-pyrazol-5(4H)-one (4). The latter was successfully used previously in mixtures with structurally related bidentate phosphine oxides 5–7 [21] (Scheme 1).
The effect of the structure of amides 1–3 on the efficiency of extraction of rare earths(III) with their mixtures with pyrazolone 4 in toluene was estimated by comparing the partition coefficients of rare earth ions (DLn) under similar experimental conditions. First of all, it was found that rare earths(III) are almost not extracted with a 0.03 M solution of 4 in toluene at pH 2 (DLn ≤10–2). Likewise, compounds 1–3 did not extract rare earths(III) under these conditions. However, the extraction efficiency significantly increased when mixtures of 1–3 and 4 were used.
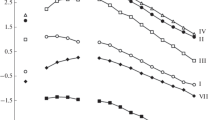
Mixtures of tridentate carbamoylmethylphosphine oxides 1–3 and pyrazolone 4 extracted rare earths(III) much more effectively than did bidentate carbamoylmethylphosphine oxide 5 (Fig. 1), which is likely to be related to the participation of an additional electron-donor P=O group of the ligand in the complexation with rare earth ions.
The difference in the extraction efficiencies of La(III) and Lu(III) with carbamoylmethylphosphine oxides 3 and 5, DLn(3)/DLn(5), decreased from 28.8 to 3.3 as the ionic radius of the rare earth element decreased. It should be noted that the length of the hydrocarbon chain on the nitrogen atom of 1–3 did not affect the extraction efficiency to an appreciable extent (Fig. 1). Presumably, the effect of steric factor hampering complexation with metal ions is compensated by increased hydrophobicity of the ligand.
The nature of organic solvent was found to strongly affect the efficiency of extraction of rare earths(III) with mixtures of 1–3 and 4. In the extraction with mixtures of 3 and 4, the DLn values increased in the series 1,2-dichloroethane < toluene < CCl4, i.e., as the solvent polarity decreased (Fig. 2). A similar pattern was observed for extraction with mixtures of chelating ligands and neutral monodentate extractants like trialkylphosphine oxides Alk3P=O [16, 24]. A considerable reduction of the extraction efficiency with the use of chloroform as solvent may be rationalized by solvation of the phosphoryl and carbonyl groups of 3 and related decrease of the concentration of the enol form of 4 in the organic phase.
It is known that rare earths(III) are extracted with solutions of 4 in inert organic solvents in the form of pyrazolates (LnP3) solvated by a molecule of 4 [18, 25], which may be illustrated by reaction (1):
Here, the subscripts “(w)” and “(o)” refer to the aqueous and organic phases, respectively, and KLn,4 is the extraction constant.
The extraction of rare earths(III) with mixtures of 1–3 and 4 involves formation of hydrophobic mixed-ligand rare earth complexes in the organic phase as a result of displacement of molecule 4 from the coordination sphere of the complex LnP3·4.
The efficiency of extraction of rare earths(III) into organic phase with a mixture of 3 and 4 increases with increase of pH of the equilibrium aqueous phase (Fig. 3). The slope of the log DLn—pH plot is close to 3 for all rare earth elements, which corresponds to transfer of three protons to the aqueous phase during the extraction process. The shift of the log DLn—pH dependence toward higher acidity of the aqueous phase for the extraction of rare earths(III) with mixtures of 3 and 4 relative to the corresponding dependence for the extraction with a solution of 4 alone indicates a significant synergistic effect in the system rare earth(III)–pyrazolone 4–ligand 3.
The stoichiometry of the extractable rare earth(III)–ligand 3 complexes was determined by the equilibrium shift method. At a constant concentration of 4 in toluene and a constant pH value of the aqueous phase, the slope of the log DLn—log[L(3)] plot is close to unity (Fig. 4), which corresponds to a ligand/metal ratio of 1 : 1. The formation of 1 : 1 complexes was also noted in the extraction of rare earths(III) with a mixture of ligand 6 and pyrazolone 4, whereas in the extraction of Eu(III) with a mixture of monodentate trioctylphosphine oxide and 4 in chloroform the extractable complex was assigned 1 : 2 Eu(III)/Oct3P=O composition [18].
At a constant concentration of phosphine oxide 3 in the organic phase and a constant pH of the aqueous phase, the slope of the log DLn—log[4] dependence is close to 3 (Fig. 5), which indicates the rare earth(III) : P– ratio 1 : 3.
With account taken of the established stoichiometry of the extractable complexes, phase distribution of rare earth ions in the extraction with mixtures of 3 and 4 in toluene can be described by Eq. (2):
Here, KLn,4,L is the extraction constant of rare earths(III) as mixed-ligand complexes. The formation of such complexes in the organic phase as a result of displacement of molecule 4 from the coordination sphere of LnP34 can be represented by Eq. (3):
where β4,L is the formation constant of the mixed-ligand complex. The quantities KLn,4,L and β4,L are related to each other through Eq. (4):
Equation (5) shows the dependence of the partition coefficient of rare earth(III) on the equilibrium concentrations of the components in the organic and aqueous phases for the extraction with mixtures of 1–3 and 4:
Table 1 contains the extraction constants of rare earths(III) with mixtures of 3 and 4 in toluene, as well as the constants of formation of mixed-ligand complexes, calculated by the least-squares method according to Eqs. (5) and (4). It is seen that the KLn,4 and β4,L values increase in parallel with the rare earth atomic number (Z). This is related to increase of the stability of rare earth complexes with hard (according to Pearson) ligands with increase in the charge density of Ln3+ ions due to reduction of their ionic radii as the atomic number increases [26]. The KLn,4,L values increase in the series from La(III) to Eu(III), and then KLn,4,L changes non-monotonically owing to tetrad effect in the extraction of rare earths(III) [27, 28]. A similar character of the dependence KLn,4,L–Z was observed for the extraction of rare earths(III) with mixtures of 4 and dibutyl{[(N,N-dibutylcarbamoyl)methoxy]methyl}phosphine oxide (7) [21].
The magnitude of the synergistic effect [SC, Eq. (6)] is determined by the stability of mixed-ligand rare earth complexes, as well as by the concentration of phosphine oxide 3 and pyrazolone 4 in the organic phase [Eq. (7)].
Here, DL, D4, and D are the partition coefficients of rare earths(III) in the extraction with phosphine oxide 3, pyrazolone 4, and their mixture, respectively.
Provided that the concentrations of 3 and 4 in the organic phase are constant, the synergistic effect decreases in the rare earth series from La(III) to Lu(III) (Table 1). A similar SC–Z dependence was typical of the extraction of rare earths(III) with mixtures of dibutyl{[(N,N-dibutylcarbamoyl)methoxy]methyl}phosphine oxide (7) [21] or phosphoryl-containing calix[6]arene [13] with pyrazolone 4.
The obtained data showed that the efficiency of extraction of rare earths(III) with tridentate carbamoylmethylphosphine oxides 1–3 considerably increases in the presence of 4-benzoyl-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one. The observed synergistic effect is related to the formation of hydrophobic mixed-ligand rare earth complexes LnP3L in the organic phase. Introduction of an additional coordinating P=O group into the amide moiety of the neutral extractant increases the extraction ability of modified tridentate carbamoylmethylphosphine oxides 1–3 in comparison to structurally related bidentate carbamoylmethylphosphine oxides. A similar effect of the structure of phosphoryl-containing extractant on the extraction efficiency was observed for di- [19] and tripodand carbamoylmethylphosphine oxides [19, 20], as well as calixarenes containing Me2P(O)CH2 groups [13].
EXPERIMENTAL
The syntheses of diphenyl{[N-alkyl-N-[(2-diphenylphosphoryl)ethyl]carbamoymethyl}phosphine oxides 1–3 [22] and diphenyl(N,N-dibutylcarbamoylmethyl)phosphine 5 [29] were described previously.
4-Benzoyl-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one of chemically pure grade (Vekton) was used without further purification. The solvents, namely chloroform, 1,2-dichloroethane, carbon tetrachloride, and toluene of chemically pure or analytical grade, were used without further purification. Solutions of extractants in organic solvents were prepared using accurately weighed samples.
Initial aqueous solutions of rare earths were prepared by dissolving the corresponding chlorides in water, followed by addition of aqueous HCl to a required concentration. A constant ionic strength (0.1 M) was maintained using sodium chloride. The concentration of rare earths in initial aqueous solutions was 2×10–6 M. All reagents were of chemically pure grade.
Extraction experiments were carried out in test tubes with ground stoppers at 22±1°C with equal volumes of the organic and aqueous phases. The phases were brought into contact using a rotary stirrer at 60 rpm for 1 h. As shown preliminarily, this time was sufficient to achieve constancy of DLn values.
The concentration of rare earths(III) in initial and equilibrium aqueous phases was determined by inductively coupled plasma mass spectrometry using a Thermo Electron X-7 instrument (USA) according to the procedure described in [30]. Their concentration in the organic phase was determined after double re-extraction with 1 M aqueous HCl. The DLn values were calculated as the concentration ratios of rare earths(III) in the organic and aqueous phases. The error in the determination of DLn did not exceed 5%. The pH values of equilibrium aqueous phases were determined using a pH 150 pH meter equipped with a glass electrode.
REFERENCES
Mikhailichenko, A.I., Mikhlin, E.B., and Patrikeev, Yu.B., Redkozemel’nye metally (Rare Earths), Moscow: Metallurgiya, 1987.
Yagodin, G.A., Kagan, S.Z., and Tarasov, V.V., Osnovy zhidkostnoi ekstraktsii (Fundamentals of Solvent Extraction), Moscow: Khimiya, 1981.
Medved’, T.Ya., Chmutova, M.K., Nesterova, N.P., Koiro, O.E., Kochetkova, N.E., Myasoedov, B.F., and Kabachnik, M.I., Bull. Acad. Sci. USSR, Div. Chem. Sci., 1981, vol. 30, no. 9, p. 1743. https://doi.org/10.1007/BF00949488
Myasoedov, B.F., Chmutova, M.K., Kochetkova, N.E., Koiro, O.E., Pribylova, G.A., Nesterova, N.P., Medved, T.Y., and Kabachnik, M.I., Solvent Extr. Ion Exch., 1986, vol. 4, no. 1, p. 61. https://doi.org/10.1080/07366298608917853
Horwitz, E.P., Martin, K.A., Diamond, H., and Kaplan, L., Solvent Extr. Ion Exch., 1986, vol. 4, no. 3, p. 449. https://doi.org/10.1080/07366298608917877
Mastryukova, T.A., Artyushin, O.I., Odinets, I.L., and Tananaev, I.G., Ross. Khim. Zh., 2005, vol. 49, no. 2, p. 86.
Turanov, A.N., Karandashev, V.K., Sharova, E.V., Artyushin, O.I., and Odinets, I.L., Tsvetn. Met., 2012, no. 3, p. 51.
Turanov, A.N., Karandashev, V.K., Kharlamov, A.V., Bondarenko, N.A., and Khvostikov, V.A., Solvent Extr. Ion Exch., 2019, vol. 37, no. 1, p. 65. https://doi.org/10.1080/07366299.2019.1592923
Bond, A.H., Dietz, M.L., and Chiarizia, R., Ind. Eng. Chem. Res., 2000, vol. 39, no 10, p. 3442. https://doi.org/10.1021/ie000356j
Atanassova, M. and Kurteva, V., RSC Adv. 2016, vol. 6, p. 11303. https://doi.org/10.1039/c5ra22306g
Atanassova, M., Kurteva, V., and Dukov, I., RSC Adv., 2016, vol. 6, p. 81250. https://doi.org/10.1039/C6RA18478B
Tashev, E., Atanassova, M., Varbanov, S., Tosheva, T., Shenkov, S., Chauvin, A.-S., and Dukov, I., Sep. Purif. Technol., 2008, vol. 64, p. 170. https://doi.org/10.1016/j.seppur.2008.09.011
Varbanov, S., Tashev, E., Vassiliev, N., Atanassova, M., Lachkova, V., Tosheva, T., Shenkov, S., and Dukov, I.L., Polyhedron, 2017, vol. 134, p. 135. https://doi.org/10.1016/j.poly.2017.06.013
Petrova, M.A., Kurteva, V.B., and Lubenov, L.A., Ind. Eng. Chem. Res., 2011, vol. 50, p. 12170. https://doi.org/10.1021/ie201207n
Pavithran, R. and Reddy, M.L.P., Anal. Chim. Acta, 2005, vol. 536, p. 219. https://doi.org/10.1016/j.aca.2004.12.042
Shmidt, V.S., Rybakov, K.A., and Rubisov, V.N., Radiokhimiya, 1982, vol. 24, no. 1, p. 25.
Rao, L., Xia, Y., Rapko, B.M., and Martin, P.F., Solvent Extr. Ion Exch., 1998, vol. 16, p. 913. https://doi.org/10.1080/07366299808934560
Santhi, P.B., Reddy, M.L.P., Ramamohan, T.R., Damodaran, A.D. Mathur, J.N., Murali, M.S., and Iyer, R.H., Solvent Extr. Ion Exch., 1994, vol. 12, no. 3, p. 633. https://doi.org/10.1080/07366299408918229
Turanov, A.N., Karandashev, V.K., Sharova, E.V., Artyushin, O.I., and Odinets, I.L., Radiochem., 2013, vol. 55, no. 2, p. 203. https://doi.org/10.1134/S1066362213020100
Turanov, A.N., Matveeva, A.G., Kudryavtsev, I.Yu., Pasechnik, M.P., Matveev, S.V., Godovikova, M.I., Baulina, T.V., Karandashev, V.K., and Brel, V.K., Polyhedron, 2019, vol. 161, p. 276. https://doi.org/10.1016/j.poly.2019.01.036
Turanov, A.N., Karandashev, V.K., Kharlamov, A.V., and Bondarenko, N.A., Solvent Extr. Ion Exch., 2014, vol. 32, no. 5, p. 492. https://doi.org/10.1080/07366299.2014.908584
Bondarenko, N.A., Belus’, S.K., Artyushin, O.I., and Peregudov, A.S., Russ. J. Gen. Chem., 2020, vol. 90, no. 12, p. 2273. https://doi.org/10.1134/S1070363220120099
Turanov, A.N., Karandashev, V.K., Artyushin, O.I., Peregudov, A.S., Khvostikov, V.A., and Bondarenko, N.A., Russ. J. Inorg. Chem., 2020, vol. 65, no. 6, p. 905. https://doi.org/10.1134/S0036023620060248
Akiba, K., Wada, M., and Kanno, T., J. Inorg. Nucl. Chem., 1981, vol. 43, no. 5, p. 1031. https://doi.org/10.1016/0022-1902(81)80169-8
Jordanov, V.M., Atanassova, M., and Dukov, I.L., Sep. Sci. Technol., 2002, vol. 37, no. 14, p. 3349. https://doi.org/10.1081/SS-120006166
Nash, K.L. and Jensen, M.P., Sep. Sci. Technol., 2001, vol. 36, nos. 5–6, p. 1257. https://doi.org/10.1081/SS-100103649
Peppard, D.F., Mason, G.W., and Lewey, S., J. Inorg. Nucl. Chem., 1969, vol. 31, no. 7, p. 2271. https://doi.org/10.1016/0022-1902(69)90044-X
Mikhailichenko, A.I., Radiokhimiya, 1975, vol. 17, no. 3, p. 352.
Turanov, A.N., Karandashev, V.K., Kharitonov, A.V., Lezhnev, A.N., Safronova, Z.V., Yarkevich, A.N., and Tsvetkov, E.N., Russ. J. Gen. Chem., 1999, vol. 69, no. 7, p. 1068.
Turanov, A.N., Karandashev, V.K., Baulin, V.E., and Tsvetkov, E.N., Russ. J. Inorg. Chem., 1995, vol. 40, no. 11, p. 1854.
Funding
This study was financially supported by the Ministry of Science and Higher Education of the Russian Federation in the framework of state assignment for Institute of Solid State Physics, Institute of Microelectronics Technology and High Purity Materials, and Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences and “Kurchatov Institute” National Research Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Translated from Zhurnal Obshchei Khimii, 2021, Vol. 91, No. 3, pp. 417–424 https://doi.org/10.31857/S0044460X21030070.
Rights and permissions
About this article
Cite this article
Turanov, A.N., Karandashev, V.K., Khvostikov, V.A. et al. Extraction of Rare Earth Elements(III) with Mixtures of Some New Tridentate Carbamoylmethylphosphine Oxides and 4-Benzoyl-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one from Hydrochloric Acid Solutions. Russ J Gen Chem 91, 383–388 (2021). https://doi.org/10.1134/S1070363221030075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221030075