Abstract
The Zn, Cd, Pb, Cu, Mn, and Fe concentrations in sediments, bottom waters, and benthic macroinvertebrates (Amphipoda, Bivalvia, Gastropoda, Hirudinea, and Oligochaeta) collected at six coastal stations in the Eastern Gulf of Finland were determined with the aim to reveal the features of heavy metals bioaccumulation in the macrozoobenthos. It was shown that benthic macroinvertebrates accumulate Mn, Fe, and Zn more actively than Pb, Cu, and Cd, while Hirudinea and Oligochaeta worms have enhanced accumulative capacities as compared to amphipods and mollusks. A close relationship was found between the Zn and Pb concentrations in the sediments and amphipods, but for the other elements no reliable relationship was observed. It was recommended to use organisms of other trophic levels along with zoobenthos and data on heavy metal concentrations in sediments for chemical monitoring of coastal waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Anthropogenic impact on aquatic ecosystems leads to a constant and steady decline in the water quality and to profound changes in the hydrological and biogeochemical cycles. Monitoring the causes and consequences of these changes is essential for diagnosing the degree of ecosystem degradation and restoration. A major threat to aquatic ecosystems is posed by heavy metals (HM). Over recent decades the release of HM into biosphere has shown an increasing trend, which generates a need for studying the processes of interaction of heavy metals with living matter. A distinctive feature of HM toxicants consists in stability and biomagnification. Toxic effect of HM is manifested at all levels of the organization of biological systems, from molecular-biochemical to biocenotic [1–3].
In the aquatic environment metal ions bind to suspended particles whose settling leads to HM accumulation in sediments. This contributes to water purification, butif background levels are significantly exceeded, sediments may become a source of secondary water pollution under certain conditions and thus constitute an environmental threat because of migration of the accumulated substances [4, 5].
Sediments provide a habitat and food source for benthic flora and fauna. Heavy metals binding to various fractions of sediments adversely affect the physiological processes in benthic organisms to the extent depending primarily on their bioavailability rather than on the total heavy metal load. Rainbow [6] considered bioaccumulation of a toxic trace metal with its uptake by the target organism as a biomonitor of trace metal bioavailability. Forms of metals present in water and sediments have different bioavailabilities; algae are sensitive to the action of dissolved forms, and zoobenthos is affected by both dissolved forms and those bound to suspended phases. Therefore, bioavailability assessment should be based on several organisms in order to cover different bioaccumulation routes [7, 8].
Bioaccumulation, or concentration, of chemical elements many of which are actively involved in various physiological processes, is one of the most important biogeochemical functions of living organisms. Demina et al. [9] consider two main types of processes of bioaccumulation of HM in marine organisms: active (metabolism) and passive (adsorption on the surface of both individual cells and whole organisms). Important active processes are bioassimilation resulting in the formation of organometallic chelate compounds and biomineralization, i.e., production of mineral forms by living organisms.
Over the years much research efforts have been focused on HM accumulation by zoobenthos, an important component of aquatic ecosystems, which displays high taxonomic diversity and capacity to accumulate HM in concentrations exceeding several orders of magnitude those observed in water [1, 9]. An essential constituent of hydrobiocenoses, zoobenthos may provide valuable information about the pollution of the environment and changes in environmental conditions, since the organisms are relatively stationary and several species live for many years [10–12]. The capacity to concentrate and tolerate high metal concentrations, along with relatively large body size and ease of collection, make benthic macroinvertebrates and their communities good bioindicators of water body pollution by heavy metals [3, 7, 13].
Bioaccumulation is influenced by both biological and geochemical factors. On the one hand, accumulation of trace elements in macrobenthic invertebrates is regulated by the input and output of metals by the organisms, which are closely related to their morphology, physiology, and metal distribution in cells, making bioaccumulation dependent on the type of the organism and its trophic level [13, 14]. On the other hand, HM bioaccumulation by zoobenthos is influenced by the nature, concentration, and bioavailability of HM, as well as by the habitat, temperature, season, diet, and other factors [11, 12, 15]. Many organisms have evolved mechanisms for regulating the concentration of trace elements in their tissues, when metal pollution is present in water, sediments, or food [16].
Though regulated by living organisms to a certain extent, the HM accumulation is not harmless [17]. Severe anthropogenic pollution causes reduction in abundance and biomass (up to complete disappearance of a number of taxa) and loss of biodiversity of zoobenthos, shell deformation, and endocrine disruptions [12, 18]. These adverse effects of HM at the organismic and population levels lie behind biomonitoring of water bodies with the use of zoobenthos. However, opinions differ regarding the use of zoobenthos in chemical monitoring (identification and quantification of pollutants) and biomonitoring (assessment of the quality of the environment). For example, mollusks have a capacity to accumulate substantial amounts of metals from ambient water, which does not always drastically affect their health. In turn, the HM content measurement in mollusks does not necessarily represent true contamination levels in the environment [19].
Bioaccumulation of toxic HM by benthic invertebrates affords removal of pollution from water bodies to an extent determined by both by the status of the ecosystem and level of anthropogenic impact [9].
The aim of this study was to identify the features of bioaccumulation of heavy metals (Zn, Cd, Pb, Cu, Mn, and Fe) by the macrozoobenthos in the coastal zone of the Eastern Gulf of Finland and to elucidate its relationship with heavy metal pollution of sediments.
To this end, the Zn, Cd, Pb, Cu, Mn, and Fe concentrations were determined in six samples of sediments and bottom water, as well as in five groups of macrozoobenthos (Amphipoda, Bivalvia, Gastropoda, Hirudinea, and Oligochaеta) associated with these sediments, bioaccumulation of Zn, Cd, Pb, Cu, Mn, and Fe in the macroinvertebrate communities was analyzed, and correlation coefficients between the concentrations of these metals in the macrozoobenthos and sediments were calculated.
EXPERIMENTAL
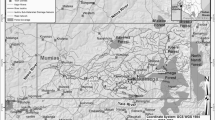
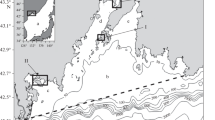
Samples of sediments and zoobenthos were collected in the shallow coastal zone of the Eastern Gulf of Finland (Fig. 1).
The sampling stations were located on the northern and southern coasts of the eastern part of the Gulf of Finland in sites facing tough environmental situation: near seaports and terminals (Primorsk, Lomonosov, Bol’shaya Izhora) and in eutrophic coastal zones with well-developed aquatic vegetation, experiencing periodic algal bloom episodes (Cape Flotsky, Sestroretsk, Grafskaya Bay).
At each station the sediment samples and water samples were collected from a depth of 50 cm at a distance of 20–30 m from the shore using a Robur-IL sampler, and zoobenthos samples with special bottom-set nets. The sediments with zoobenthos were washed through a sieve (0.333 mm mesh), fixed with 4% formalin solution, and stored in sealed plastic bags. Benthic invertebrate specimens were taken from the washed sediment in the laboratory setting under a microscope and classified into systematic groups.
In this study we assessed five benthic invertebrate communities (Amphipoda, Bivalvia, Gastropoda, Hirudinea, and Oligochaeta) for the ability to accumulate HM.
Amphipoda (sideswimmers) is an order of Malacostarcan crustaceans (Peracarida superorder). Having significantly increased in number of species and abundance duringrecent years due to the newly introduced species, Amphipoda account today for 41% of the total biomass of macrozoobenthos in the ecosystem of the Gulf of Finland. Amphipods are omnivores possessing mixed feeding, major consumers of plant detritus; adults are active predators [20]. Bivalvia (bivalve mollusks) are a class of mollusks employing sedentary lifestyle, filter feeders for the most part. Gastropoda (gastropods or snails) are the most highly diversified class of the phylum Mollusca having extremely varied feeding habits(they feed both on living plants and detritus). Hirudinea (leeches) and Oligochaeta (small-bristle worms) are a subclass of annelids from the class of girdle worms. Hirudinea are highly modified descendants of oligochaetes, feeding on animal blood; they can attack mollusks and other invertebrates. Oligochaeta feed on detritus which they absorb with the soil. Thus, according to their diet, the above-listed benthic macroinvertebrates belong to different groups, including filter feeders, detritivores, euryphages, scavengers, predators, and ectoparasites.
The sediment samples collected were dried at 30°C and sifted through a plastic sieve with a pore diameter of 1 mm. The sifted out fraction was ground in an agate mortar, whereupon a weighted quantity of the sediments was placed in Teflon beakers and treated with a mixture of HCl/HF/HNO3 acids (1 : 1 : 1) in a Mars 5 microwave system (CEM, the United States). Mineralizates of the sediments thus decomposed were evaporated, filtered, transferred to volumetric flasks, and made up to 25 mL with deionized water. Next, the gross Zn, Cd, Pb, Cu, Mn, and Fe concentrations in the salt solutions after sample preparation were determined by inductively coupled plasma mass spectrometry (ICP-MS) on an Agilent 7700x instrument (Agilent Technologies, Japan). The same technique was employed for sample preparation and analysis for the Zn, Cd, Pb, Cu, Mn, and Fe content in the zoobenthos. Water samples were analyzed by ICP-MS as well. All the analyses were carried out at least in triplicate; relative root mean square errors of the analyses did not exceed 15%. Validity of the analyses was guaranteed by carrying out blank experiments and by using state standard reference sample.
RESULTS AND DISCUSSION
Granulometric analysis showed that the content of the sand fraction in the upper layer of the coastal sediments of the Gulf of Finland was 90–99%, with the organic matter content not exceeding 0.8% and quartz with minor silicate impurities being the main phase [21]. Because of a high content of the sand fraction the sorption capacity of the sediments with respect to HM was many times lower than that of natural soil sorbents, 4–10 μmol/g.
Table 1 and 2 list the average Zn, Cd, Pb, Cu, Mn, and Fe concentrations in the samples of the coastal sediments and water.
The average metal concentrations in the coastal sediments of the Gulf of Finland vary as Cd < Cu < Pb << Zn < Mn < Fe. This series is somewhat different from that for the average metal concentrations in water: Cd < Pb < Zn < Cu < Mn < Fe (Tables 1 and 2). The HM concentrations in the coastal zone typically exceed those in the open-water zones due to anthropogenic and natural factors (surface and river runoffs, abrasion) [22]. However, assessment of the ecological risk of the HM accumulation in the coastal sediments the Gulf of Finland showed that the pollution of this part of the Gulf of Finland by HM was generally below hazardous levels. This may be related to poor sorption capacity of the coastal sediments. Though found in low concentrations, Cd poses the greatest environmental threat to aquatic ecosystems [23].
Table 3 lists the metal concentrations detected in five benthic invertebrate species (crustaceans, bivalve mollusks, gastropods, leeches, and small-bristle worms) from the surface sediments collected at the stations indicated.
Total concentrations of each of the metals in the whole body (including soft tissues and shells) were determined for all the benthic macroinvertebrate communities studied. These data take into account the weight contribution of the shells whose biomass concentrates many toxic metals and thus more accurately reflect the real accumulation of metals [24]. The HM concentrations in the zoobenthos varied over four orders of magnitude, from 0.1–0.5 μg/g (Cd) to 500–5000 μg/g (Fe) (Table 3).
The average HM concentrations in the coastal macrozoobenthos decreased as Cd < Pb < Cu < Zn < Mn < Fe (Table 4), which series correlates to varying degrees with both the HM content in the sediments and HM bioavailability and biological demand. This sequence is typical not only for macrozoobenthos as a whole but also for each community studied in particular.
The ability of benthic invertebrates to concentrate metals was quantified through the bioconcentration factors (BCFwaterr) (Table 5), which were taken to be equal to the ratios of the content of an element in the organism to that in bottom water [9].
The heavy metal bioconcentration series in which the metals were arranged in accordance with their corresponding BCFwater values differ insignificantly among the zoobenthos communities of interest; the ability of benthic macroinvertebrates to concentrate HM, in general, decreases as Mn > Fe ≥ Zn > Pb> Cu ≥ Cd. Thus, essential trace elements Mn, Fe, and Zn are quite predictably accumulated in the macroinvertebrates more actively than Cd, Cu, and Pb. High bioconcentration factors revealed for Mn are probably attributable to high bioavailability of this metal. The highest concentrations of all the elements (except for Cu) among the benthic macroinvertebrates of interest were found in Hirudinea and Oligochaeta worms (Table 3), which may be due to their peculiar physiology, in particular, underdeveloped organs responsible for excretion of hazardous elements. High bioconcentration factors of Cu in the amphipods (BCFwater = 18×103) and of Zn in the leeches (BCFwater = 220×103) deserve mentioning.
The total capacity of a species to concentrate trace elements was quantified through the biogeochemical activity of species (BCA) which is defined as the sum of the bioconcentration factors of individual trace elements as proposed by A.D. Aivazyan [25].
Our studies revealed high biogeochemical activity in Hirudinea and Oligochaeta worms [BCA = (400‒600)× 103] and lower and practically identical biogeochemical activities in mollusks and crustaceans [BCA = (210‒230)×103 ] (Table 5). These findings are consistent with high sensitivity of oligochaetes and leeches from the Taihu Basin (China) to heavy metals contamination of sediments and with enhanced metal bioaccumulation capacity compared to other macroinvertebrates, as reported by Bian et al. [3].
Bottom invertebrate communities, preferentially indigenous species, are of frequent use as bioindicators of pollution of the coastal and estuarine environments. Considering relative simplicity of collecting crustaceans as compared to gastropods and leeches, high HM bioaccumulative capacity, and domination in the macrozoobenthos community of the coastal area of the Eastern Gulf of Finland [20], we selected amphipods for our biomonitoring studies.
To establish the relationship between the level of HM pollution of the coastal water areas and the HM accumulation by amphipods, Pearson’s correlation coefficients (n = 6, p ≤ 0.05) were calculated between the Zn, Cd, Pb, Cu, Mn, and Fe concentrations in the sediments and in the amphipods collected at the same coastal stations (Table 6).
Fairly high correlation coefficients (see Table 6) suggest a strong relationship between the Zn and Pb concentrations in the sediments and in the amphipods and a weak relationship for other elements. Low correlation coefficients in the case of Mn and Fe are apparently attributable to high contents of these elements in the sediments, little comparable to the amount of manganese and iron required by zoobenthos.
Our data evidence that use of amphipods as bioindicators is not good enough for chemical monitoring of coastal water areas. Lack of statistically significant correlation between the metal concentrations in macrozoobenthos and sediments was noted in a number of studies [26–29].
Presumably, along with various benthic macroinvertebrate species, hydrobionts of other trophic levels should be involved as bioindicators. For example, periphyton algae Cladophora glomerata was recommended as a bioindicator to monitor the HM pollution of the coastal area of the Eastern Gulf of Finland [30]. Bioaccumulation (the content of elements in living organisms) does not necessarily reflect the environmental metal concentrations and thus should be used in conjugation with data on the concentrations of metals in abiotic components of the ecosystem.
CONCLUSIONS
Specific features of heavy metals bioaccumulation by benthic invertebrates from the coastal zone of the Eastern Gulf of Finland (amphipods, bivalve mollusks, gastropods, leeches, and small-bristle worms) were revealed. The Zn, Cd, Pb, Cu, Mn, and Fe concentrations in the sediments, bottom waters, and macrozoobenthos samples collected at six coastal stations located on the northern and southern sides of the Gulf of Finland were determined. The average HM concentrations in the coastal macrozoobenthos were recorded in ascending order as Cd < Pb < Cu < Zn < Mn < Fe. Amphipods displayed enhanced capacity for Cu accumulation. Among the benthic macroinvertebrates studied, Hirudinea and Oligochaeta worms exhibited the highest concentrations of the other elements. Essential microelements Mn, Fe, and Zn were quite predictably accumulated by the macroinvertebrates much more actively than Pb, Cu, and Cd. The biogeochemical activity indicators revealed for the leeches and small-bristle worms were almost twice as large as those for the mollusks and crustaceans. Pearson’s correlation coefficients between the Zn, Cd, Pb, Cu, Mn, and Fe concentrations in the sediments and amphipods are indicative of a reliable relationship for Zn and Pb (correlation coefficients 0.99 and 0.78, respectively), but for the other elements no reliable relationship was observed. Along with various macrozoobenthos species, bioindicator organisms of other trophic levels, as well as data on the metal concentrations in sediments should be used for chemical monitoring of water bodies.
REFERENCES
Brown, M.T. and Depledge, M.H., Metabolism of Trace Metals in Aquatic Organisms, Bebianno, M.J., and Langston, W.J., Eds., London: Chapman & Hall, 1998, p. 185.
Chiarelli, R. and Roccheri, M.C., Open J. Metal, 2014, vol. 4, no. 4, p. 93. https://doi.org/10.4236/ojmetal.2014.44011
Bian, B., Zhou, Y., and Fang, B.B., Ecol. Indic., 2016, vol. 69, p. 348. https://doi.org/10.1016/j.ecolind.2016.04.048
Linnik, P.N., Zhezherya, V.A., and Zhezherya, T.P., Ekol. Khim., 2016, vol. 25, no. 4, p. 222.
Yujun, Y., Zhifeng, Y., and Shanghong, Z., Environ. Pollut., 2011, vol. 159, no. 10, p. 2575. https://doi.org/10.1016/j.envpol.2011.06.011
Rainbow, P.S., Mar. Pollut. Bull., 1995, vol. 31, nos. 4‒12, p. 183. https://doi.org/10.1016/0025-326X(95)00116-5
Amado-Filho, G.M., Salgado, L.T., Rebelo, M.F., Rezende, C.E., Karez, C.S, and Pfeiffer, W.C., Braz. J. Biol., 2008, vol. 68, no. 1, p. 95. https://doi.org/10.1590/s1519-69842008000100013
Rodriguez, P., Mendez-Fernandez, L., Pardo, I., Costas, N., and Martinez-Madrid, M., Ecol. Indic., 2018, vol. 91, p. 395. https://doi.org/10.1016/j.ecolind.2018.04.004
Demina, L.L., Galkin, S.V., and Bud’ko, D.F., Materialy XXI Mezhdunarodnoi konferentsii (Shkoly) po morskoi geologii “Geologiya morei i okeanov” [Proc. XXI Int. Conf. (School) on Marine Geology “Geology of Seas and Oceans”], Moscow, November 16–20, 2015, vol. IV, Moscow, 2015, p. 23.
Boening, D.W., Environ. Monit. Assess, 1999, vol. 55, p. 459. https://doi.org/10.1023/A:1005995217901
Webb, A.L., Hughes, K.A., Granda, M.M., Lohana, M.C., and Peck, L.S., Sci. Total Environ., 2020, vol. 698, Art. 134268. https://doi.org/10.1016/j.scitotenv.2019.134268
Dar, M.A., Belal, A.A., and Madkour, A.G., Egypt. J. Aquat. Res., 2018, vol. 44, no. 4, p. 291. https://doi.org/10.1016/j.ejar.2018.11.008
Kiffney, P.M. and Clements, W.H., Environ. Toxicol. Chem., 1993, vol. 1, no. 8, p. 1507.
Pastorino, P., Pizzul, E., Bertoli, M., Perilli, S., Brizio, P., Salvi, G., Esposito, G., Abete, M.C., Prearo, M., and Squadrone, S., Environ. Sci. Pollut. Res., 2020, vol. 27, p. 5958. doi: 10.1007/s11356-019-07325-x
Pastorino, P., Bertoli, M., Squadrone, S., Brizio, P., Piazza, G., Noser, A.G.O., Prearo, M., Abete, M.C., and Pizzul, E., Ecohydrol. Hydrobiol., 2019, vol. 19, no. 3, p. 428. https://doi.org/10.1016/j.ecohyd.2019.04.006
Kalantzi, I., Papageorgiou, N., Sevastou, K., Black, K.D., Pergantis, S.A., and Karakassis, I., Sci. Total Environ., 2014, vols. 470‒471, p. 742. https://doi.org/10.1016/j.scitotenv.2013.10.020
Kesavan, K., Murugan, A., Venkatesan, V., and Vijay Kumar, B.S., Thalassas, 2013, vol. 29, no. 2, p.15.
Takarina, N.D. and Adiwibowo, A., J. Coast. Dev., 2011, vol. 14, no. 2, p. 168.
Zuykov, M., Pelletier, E., and Harper, D.A.T., Chemosphere, 2013, vol. 93, no. 2, p. 201. https://doi.org/10.1016/j.chemosphere.2013.05.001
Berezina, N.A. and Maksimov, A.A., Zh. Sib. Fed. Univ.: Biol., 2016, vol. 9, no. 4, p. 409.
Polyak, Yu.M., Shigaeva, T.D., Kudryavtseva, V.A., and Konakov, V.G., Voda: Khim. Ekol., 2017, no. 1, p. 11.
Bud’ko, D.F. and Martynova, D.M., Okeanologiya, 2019, vol. 59, no. 1, p. 33. https://doi.org/10.31857/S0030-15745933-44
Levit, R.L. and Popova, T.A., Voda: Khim. Ekol., 2019, nos. 1‒2, p. 3.
Demina, L.L., Gordeev, V.V., Galkin, S.V., Kravchishina, M.D., and Aleksankina, S.P., Okeanologiya, 2010, vol. 50, no. 5, p. 771.
Dobrovol’skii, V.V., Osnovy biogeokhimii (Fundamentals of Biogeochemistry), Moscow: Akademiya, 2003.
Kong, M., Hang, X., Wang, L., Yin, H., and Zhang, Y., Water Sci. Technol., 2016, vol. 73, no. 1, p. 203. https://doi.org/10.2166/wst.2015.483
Fan, W., Ren, J., Wu, C., Tan, C., Wang, X., Cui, M., Wu, K., and Li, X., Environ. Sci. Pollut. Res., 2014, vol. 21, no. 24, p. 14069. https://doi.org/10.1007/s11356-014-3325-6
Kholodkevich, S.V., Sharov, A.N., Chuiko, G.M., Kuznetsova, T.V., Gapeeva, M.V., and Lozhkina, R.A., Vodn. Resur., 2019, vol. 46, no. 2, p. 214. https://doi.org/10.31857/S0321-0596462214-224
Søberg, L.C., Vollertsen, J., Blecken, G.-T., Nielsen, A.H., and Viklander, M., Urb. Water J., 2016, vol. 13, no. 7, p. 697. https://doi.org/10.1080/1573062X.2015.1024689
Gubelit, Y., Polyak, Y., Dembska, G., PazikowskaSapota, G., Zegarowski, L., Kochura, D., Krivorotov, D., Podgornaya, E., Burova, O., and Maazouzi, C., Sci. Total Environ., 2016, vol. 550, p. 806. https://doi.org/10.1016/j.scitotenv.2016.01.122
ACKNOWLEDGMENTS
We are grateful to A.S. Demchuk, Junior Researcher of the Zoological Institute, Russian Academy of Sciences, for the collection and classification of the zoobenthos.
Funding
This study was carried out within the framework of the State assignment on the theme of the St. Petersburg Federal Research Center of the Russian Academy of Sciences “Development of Methods for Early Diagnosis and Prevention of Threats to the Ecological Safety of Ecosystems in the Northwest of Russia” (state registration no. AAAA-A19-119020190122-6).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Levit, R.L., Shigaeva, T.D. & Kudryavtseva, V.A. Heavy Metals in Macrozoobenthos and Sediments of the Coastal Zone of the Eastern Gulf of Finland. Russ J Gen Chem 90, 2700–2707 (2020). https://doi.org/10.1134/S1070363220130265
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220130265