Abstract
The reaction of triphenylcyclopentadienyl potassium with praseodymium and erbium chloride tetrahydrofuranates gives, depending on the reactant ratio, tetranuclear ate complexes, [{(Ph3C5H2)-Pr(THF)}2(µ2-Cl)2(µ3-Cl)3K]2(C7H8)4 (I) and [{(Ph3C5H2)Er(THF)}2(µ2-Cl)2(µ3-Cl)3K(THF)]2 (III), or binuclear ate complexes [(Ph3C5H2)2LnCl(KCl)]2 Ln = Pr (II), Er (IV) (CCDC nos. 2224244 (I), 2224243 (II), 2224245 (III), 2224242 (IV)). The structurally similar complexes I and III are based on the {[Ln2(µ-Cl)3]2(µ-Cl)2K2} core, and in III, the potassium cation is additionally coordinated to the THF molecule. The isostructural complexes II and IV have the binuclear [Ln(µ-Cl)2K]2 core.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Substituted cyclopentadienyl ligands play an important role in the design and synthesis of organometallic compounds of 4f elements [1–4]. The large variety of these ligands and, simultaneously, the interest in these ligands is due to the ease of modification of the cyclopentadienyl moiety. Aryl-substituted cyclopentadienyl ligands are especially promising in this respect, since they can be modified both by using different numbers of substituents in the cyclopentadienyl ring and by introducing substituents into the aryl moiety [5–8]. Previously, we reported the synthesis of a series of polyphenyl-substituted gadolinium, neodymium, and terbium cyclopentadienyl ate complexes. In the series of mono- and bis-triphenylcyclopentadienyl complexes, two stable structural motifs were found: the binuclear [Ln(µ-Cl)2K]2 core for the bis-cyclopentadienyl complexes [\({\text{Cp}}_{{\text{2}}}^{{{\text{Ph3}}}}\)Ln(µ2-Cl)(µ3-Cl)K(THF)n]2, n = 0 (Ln = Gd, Tb), n = 2 (Ln = Nd), and the tetranuclear {[Ln2(µ-Cl)4]2(µ-Cl)2K2} core for the monocyclopentadienyl complexes {[CpPh3Ln-(THF)]2(µ2-Cl)2(µ3-Cl)3K(THF)n}2, n = 0 (Ln = Nd), 1 (Ln = Gd, Tb) [9–11].
It appeared of interest to study the structural features of analogous triphenylcyclopentadienyl complexes of lanthanides located in the middle of the 4f series with ionic radius either greater or smaller than the terbium and gadolinium radii. It was assumed that movement towards the beginning and/or end of the 4f series, accompanied by the corresponding change in the ionic radius, would result in rearrangement of the coordination sphere of the lanthanide ions in these complexes.
The purpose of this work is to synthesize and study the structures of erbium and praseodymium mono- and bis-triphenylcyclopentadienyl complexes and to compare their structural features with those of related compounds of terbium, gadolinium, and neodymium.
EXPERIMENTAL
Compounds I–IV were synthesized in a prepurified argon atmosphere using anhydrous solvents and a SPEKS-GB2 glove box. Tetrahydrofuran was pre-dried over NaOH and distilled from potassium/benzophenone. Hexane was distilled from potassium sodium eutectics/benzophenone. Toluene was distilled from sodium /benzophenone. PrCl3(THF)2 and ErCl3(THF)3 were obtained by a known procedure [12]. Benzyl potassium was prepared according to a modified published procedure [13]. 1,2,4-Triphenylcyclopentadiene was synthesized by a reported procedure [14], recrystallized from absolute ethanol, and dried in a dynamic vacuum. Elemental analysis of complexes I–III was carried out on a Thermo Scientific FLASH 2000 CHNS/O Analyzer. Elemental analysis of complex IV was performed on a Perkin-Elmer 24000 Series II elemental CHNS/O instrument. The metal content was determined by complexometric titration with EDTA using xylenol orange indicator.
Synthesis of [{(Ph3C5H2)Pr(THF)}2(µ2-Cl)2(µ3-Cl)3K]2(C7H8)4 (I). A solution of benzyl potassium (0.265 g, 2.04 mmol) in THF (5 mL) was slowly added with stirring to 10 mL of a THF solution of 1,2,4-triphenylcyclopentadiene (0.588 g, 2 mmol). The reaction mixture was stirred for 15 min, the resulting solution of 1,2,4-triphenylcyclopentadienyl potassium was slowly added to a stirred suspension of PrCl3(THF)2 (0.783 g, 2 mmol) in THF (6 mL). The reaction mixture was stirred for 12 h, then centrifuged to separate the precipitated potassium chloride. The precipitate was washed with THF (5 mL) and centrifuged once again. The supernatants were combined and evaporated to dryness. The resulting viscous oil was dissolved in toluene (15 mL), and hexane (20 mL) was carefully added to the solution, while avoiding mixing of the phases. After 10 days, bright green crystals of complex I were formed. The crystals were dried in a dynamic vacuum. The yield of complex I was 0.764 g (0.289 mmol, 58%).
For C122H116O4Cl10K2Pr4 | |||
Anal. calcd., % | C, 55.45 | H, 4.43 | Pr, 21.35 |
Found, % | C, 55.70 | H, 4.65 | Pr, 20.87 |
The crystals suitable for X-ray diffraction were obtained by slow diffusion of hexane into a solution of I in toluene. According to X-ray diffraction data, the unit cell of complex I contained four toluene molecules. Two of these molecules were lost during vacuum drying.
Synthesis of [(Ph3C5H2)2PrCl(KCl)]2 (II) was performed by a procedure similar to that used for I starting from benzyl potassium (0.530 g, 4.08 mmol), 1,2,4-triphenylcyclopentadiene (1.176 g, 4 mmol), and PrCl3(THF)2 (0.783 g, 2 mmol). The yield of II was 0.787 g (0.470 mmol, 47%).
For C92H68Cl4K2Pr2 | |||
Anal. calcd., % | C, 65.96 | H, 4.10 | Pr, 16.84 |
Found, % | C, 66.05 | H, 4.19 | Pr, 16.99 |
Synthesis of [{(Ph3C5H2)Er(THF)}2(µ2-Cl)2(µ3-Cl)3K(THF)]2 (III) was performed by a procedure similar to that used for I starting from benzyl potassium (0.265 g, 2.04 mmol), 1,2,4-triphenylcyclopentadiene (0.588 g, 2 mmol), and ErCl3(THF)3 (0.979 g, 2 mmol). The yield of complex III was 0.641 g (0.237 mmol, 47%).
For C116H116O6Cl10K2Er4 | ||
Anal. calcd., % | C, 51.45 | H, 4.32 |
Found, % | C, 51.75 | H, 4.34 |
The crystals suitable for X-ray diffraction were obtained by slow diffusion of hexane into a solution of III in THF.
Synthesis of [(Ph3C5H2)2ErCl(KCl)]2 (IV) was performed by a procedure similar to that used for I starting from benzyl potassium (0.265 g, 2.04 mmol), 1,2,4-triphenylcyclopentadiene (0.588 g, 2 mmol), and ErCl3(THF)3 (0.490 g, 1 mmol). For the isolation of IV, the viscous oil formed upon evaporation of the reaction mixture after centrifugation was washed with toluene (10 mL), and the pink precipitate was separated by centrifugation and dissolved in THF (15 mL). Hexane (20 mL) was carefully added to the solution, while avoiding mixing of the phases. After 10 days, pink crystals formed. After vacuum drying, the yield of the crystals was 0.062 g. One more portion of hexane (20 mL) was added to the mother liquor; this additionally gave 0.139 g of IV. The total yield of IV was 0.201 g (0.201 mmol, 23%).
For C92H68Cl4K2Er2 | ||
Anal. calcd., % | C, 63.95 | H, 3.97 |
Found, % | C, 62.94 | H, 4.09 |
The crystals suitable for X-ray diffraction were obtained by slow diffusion of hexane into a solution of IV in THF. The low elemental analysis data and a low yield of complex IV suggest that the isolated single crystalline sample was not the major product of this reaction, but only one of several products. Indeed, when the synthesis of IV was reproduced, 1,2,4-triphenylcyclopentadienylpotassium solvate was isolated in some cases in the crystalline state from the reaction mixture.
X-ray diffraction study of complexes I–IV was carried out on a Bruker Quest D8 diffractometer (Photon-III detector, MoKα radiation, graphite monochromator, ω-scan mode). The reflection intensities were obtained using the SAINT software [15]. The absorption corrections were applied semiempirically on the basis of equivalent reflections in the SADABS software [16]. The structures were solved by direct methods using the SHELXT software [17] and refined by the least-squares method in the anisotropic full-matrix approximation on \(F_{{hkl}}^{2}\) using the SHELXL-2018 software [18]. In the refining of disordered groups, constraints on the atom displacement parameters and positional parameters (DFIX and EADP) were used. The hydrogen atoms in all structures were located by the rigid-body model (C–H distance is 0.950 Å for aromatic, 0.990 Å for methylene, and 1.000 Å for cyclopentadienyl hydrogen atoms) and refined in the relative isotropic approximation Uiso(H) = 1.2Uequiv(C). The main crystallographic data and refinement parameters for compounds I–IV are summarized in Table 1.
The atom coordinates and other parameters of structures of I–IV were deposited with the Cambridge Crystallographic Data Centre (CCDC no. 2224242–2224245, deposit@ccdc.cam.ac.uk or http://www. ccdc.cam.ac.uk/data_request/cif).
RESULTS AND DISCUSSION
Treatment of a suspension of praseodymium chloride tetrahydrofuranate PrCl3(THF)2 in THF with a solution of 1,2,4-triphenylcyclopentadienylpotassium in THF resulted, depending on the reactant ratio, in the formation of ate complexes [{CpPh3Pr(THF)}2(µ2-Cl)2(µ3-Cl)3K]2 (I) or [\({\text{Cp}}_{{\text{2}}}^{{{\text{Ph3}}}}\)PrCl(KCl)]2 (II) (THF is tetrahydrofuran, CpPh3 = 1,2,4-triphenylcyclopentadienyl) (Scheme 1).

Scheme 1 .
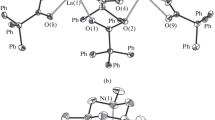
The structures of the products were established by X-ray diffraction. Complex I consists of two equivalent [{CpPh3Pr(THF)}2(µ2-Cl)2(µ3-Cl)3K] moieties combined by two K–Cl bonds (Fig. 1). Complex I is isostructural to the related neodymium complex [{CpPh3Nd(THF)}2(µ2-Cl)2(µ3-Cl)3K]2 [11], but differs from the gadolinium and terbium triphenylcyclopentadienyl complexes [{CpPh3Ln(THF)}2(µ2-Cl)2(µ3-Cl)3K(THF)]2 [9] (Ln = Gd, Tb) in the coordination environment of potassium ions. In complex I, the potassium ion is coordinated by four µ3-bridging and one µ2-bridging chlorine atoms and is η6-coordinated by the phenyl substituent of one cyclopentadienyl ligand. Meanwhile, in [{CpPh3Ln(THF)}2(µ2-Cl)2(µ3-Cl)3K(THF)]2, the potassium ion is coordinated, along with five chloride ligands, by the THF oxygen atom and is also η2-coordinated by the phenyl group.
Each of the two non-equivalent praseodymium cations in I is coordinated by four bridging chloride ligands, the THF oxygen atom, and the η5-cyclopentadienyl anion (coordination number 8). The rotation angles of the phenyl groups relative to the cyclopentadienyl ring range from 8.7° to 44.9°; furthermore, the rotation angles of the phenyl groups in position 4 of the cyclopentadienyl anion are markedly smaller than those for the phenyl groups in positions 1 and 2 (see Table 2).
It is of interest that almost all lanthanide monoarylcyclopentadienyl complexes containing no other ligands except the cyclopentadienyl and halide ligands have the [M4K2Cl10] structural motif [9, 11, 19–21]. The only exception is the [CpPh3YCl2(THF)3] complex, which is mononuclear [22]. An example of such structure is also known in the d-metal chemistry [23].
Bis-cyclopentadienyl complex II, like complex I, is located at the inversion center (Fig. 2). It refers to the ate complex structural type unusual for organolanthanide chemistry in which the alkali metal cation is not coordinated by the O- or N-donor ligand: [Cp2LnX2M], where Cp is substituted or unsubstituted cyclopentadienyl, X is an anionic ligand, and M is an alkali metal cation. For praseodymium, complexes of this type have been unknown before.
In complex II, praseodymium is coordinated by two η5-cyclopentadienyl ligands and two chloride ligands (coordination number 8), as in complex I. The Pr–\({\text{Cp}}_{{{\text{centroid}}}}^{{{\text{Ph3}}}}\) distance is somewhat shorter in the bis-cyclopentadienyl complex (on average, 2.498 Å) than in the monocyclopentadienyl complex (on average, 2.510 Å). At the same time, the average rotation angles of the phenyl rings relative to the cyclopentadienyl ring are much greater in II than in I. The average values of the rotation angles are 38.1° (II) and 29.3° (I) for the phenyl groups in positions 1 and 2 of the cyclopentadienyl ligand and 18.9° (II) and 11.7° (I) for those in position 4. This considerable difference may be indicative of greater steric crowding of praseodymium in complex II in comparison with complex I.
Complex II is isostructural to the previously described related compounds of gadolinium and terbium [9].
The erbium complexes [{CpPh3Er(THF)}2(µ2-Cl)2(µ3-Cl)3K(THF)]2 (III) and [Cp\(_{2}^{{{\text{Ph3}}}}\)ErCl(KCl)]2 (IV) were prepared by analogy with praseodymium complexes (Scheme 2). Complexes III and IV were isolated by recrystallization from THF–hexane mixtures.

Scheme 2 .
The structure of complex III was also established by X-ray diffraction analysis. Complex III (Fig. 3) is structurally similar to I. The key difference between the structures of I and III is in the coordination environment of the potassium ion. In praseodymium complex I, the potassium ion is coordinated by five chloride ligands and the π-system of one of the phenyl substituents in triphenylcyclopentadienyl; in erbium complex III, the potassium ion is coordinated by five chloride ligands and a tetrahydrofuran molecule. Complex III is isostructural to the gadolinium and terbium complexes [{CpPh3Ln(THF)}2(µ2-Cl)2(µ3-Cl)3K(THF)]2 [9] (Ln = Gd, Tb), which actually was to be expected in view of the similarity of the Gd3+, Tb3+, and Er3+ ionic radii.
The structure of IV (Fig. 4) was also established by X-ray diffraction. According to the results, complex IV is isostructural to II and to related terbium and gadolinium complexes [20].
In this study, we prepared and structurally characterized erbium and praseodymium mono- and bis-triphenylcyclopentadienyl chloride complexes. All of the obtained compounds are ate complexes. Praseodymium monocyclopentadienyl complex I is isostructural to the related neodymium complex, erbium complex III is isostructural to terbium and gadolinium complexes, while bis-cyclopentadienyl complexes of praseodymium II and erbium IV are isostructural to analogous terbium and gadolinium bis-cyclopentadienyl complexes.
REFERENCES
Arndt, S. and Okuda, J., Chem. Rev., 2002, vol. 102, no. 6, p. 1953.
Edelmann, F.T., Comprehensive Organometallic Chemistry III, Elsevier, 2007, p. 1.
Day, B.M., Guo, F.S., and Layfield, R.A., Acc. Chem. Res., 2018, vol. 51, no. 8, p. 1880.
Evans, W.J. and Davis, B.L., Chem. Rev., 2002, vol. 102, no. 6, p. 2119.
Xu, J., Gao, W., Zhang, Y., et al., J. Organomet. Chem., 2007, vol. 692, no. 1, p. 1505.
Ye, J., Deng, D., Gao, Y., et al., Spectrochim. Acta. A. Mol. Biomol. Spectrosc., 2015, vol. 134, p. 22.
Zhang, X., Ye, J., Xu, L., et al., J. Lumin., 2013, vol. 139, p. 28.
Yang, L., Ye, J., Xu, L., et al., RSC Adv., 2012, vol. 2, no. 30, p. 11529.
Roitershtein, D.M., Puntus, L.N., Vinogradov, A.A., et al., Inorg. Chem., 2018, vol. 57, no. 16, p. 10199.
Minyaev, M.E., Komarov, P.D., Roitershtein, D.M., et al., Organometallics, 2019, vol. 38, no. 15, p. 2892.
Minyaev, M.E., Vinogradov, A.A., Roitershtein, D.M., et al., J. Organomet. Chem., 2016, vol. 818, p. 128.
Edelmann, F.T. and Poremba, P., Synthetic Methods of Organometallic and Inorganic Chemistry (Herrman/Brauer), Edelmann, F.T. and Herrmann, W.A., Eds., Stuttgart, 1997, p. 34.
Lochmann, L. and Trekoval, J., J. Organomet. Chem., 1987, vol. 326, no. 1, p. 1.
Hirsch, S.S. and Bailey, W.J.J., Org. Chem., 1978, vol. 43, no. 21, p. 4090.
APEX-III, Madison: Bruker AXS Inc., 2019.
Krause, L., Herbst-Irmer, R., Sheldrick, G.M., and Stalke, D., J. Appl. Crystallogr., 2015, vol. 48, p. 3.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Adv., 2015, vol. 71, p. 3.
Sheldrick, G.M., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, p. 3.
Bardonov, D.A., Lysenko, K.A., Nifant’ev, I.E., and Roitershtein, D.M., Russ. J. Coord. Chem., 2022, vol. 48, no. 5, p. 295. https://doi.org/10.1134/S1070328422050013
Vinogradov, A.A., Komarov, P.D., Puntus, L.N., et al., Inorg. Chim. Acta, 2022, vol. 533, no. 120777.
Komarov, P.D., Nifant’ev, I.E., Roitershtein, D.M., and Minyaev, M.E., J. Chem. Crystallogr., 2021, vol. 51, p. 352.
Roitershtein, D.M., Minyaev, M.E., Mikhaylyuk, A.A., et al., Russ. Chem. Bull., 2012, vol. 61, p. 1726.
Fohlmeister, L. and Jones, C., J. Chem. Crystallogr., 2014, vol. 44, p. 301.
Funding
This study was supported by the Russian Science Foundation (grant no. 22-13-00312).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Degtyareva, S.S., Bardonov, D.A., Lysenko, K.A. et al. Praseodymium and Erbium 1,2,4-Triphenylcyclopentadienyl Complexes. Russ J Coord Chem 49, 513–520 (2023). https://doi.org/10.1134/S107032842370063X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107032842370063X