Abstract
The reaction of manganese(II) acetate hydrate with cymantrenecarboxylic acid under inert atmosphere gave the complex [Mn(Thf)2(OH2)4][OOCC5H4Mn(CO)3]2 (I), which was highly unstable to air oxygen and temperature of the adduct, in which the anions occupy the outer-sphere positions. The oxidation of the mother liquor after isolation of the single crystals of I afforded the complex Mn6(µ4-O)2[µ,η2-OOCC5H4Mn(CO)3]2[µ-OOCC5H4Mn(CO)3]8(OH2)4·5C6H6·THF·3H2O (II). According to X-ray diffraction data, the metal core of II was a hexanuclear cluster \({\text{Mn}}_{4}^{{{\text{II}}}}{\text{Mn}}_{2}^{{{\text{III}}}}\) containing mixed-valence metal atoms. Apart from X-ray diffraction, the obtained unstable complexes were characterized by elemental analysis and IR spectroscopy (powders).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Organic peroxides formed upon the reactions of some solvents (tetrahydrofuran, ethers, etc.) with air oxygen in the light may act, similarly to hydrogen peroxide, as convenient oxidants for metal complexes, giving rise to oxo- and hydroxo-bridged polynuclear compounds and clusters [1, 2].
For example, in the case of cobalt(II) pivalates, oxidation with air oxygen was found for a dibenzyl ether solution of \({\text{Co}}_{3}^{{{\text{II}}}}\)(μ-OOCtBu)6(NEt3)2 to give the cluster [\({\text{Co}}_{6}^{{{\text{III}}}}\)(µ4-O)2(µ3-O)2(µ-OOCtBu)9(OH)2(HOOCtBu)]-(HNEt3) and for a THF solution of the violet product of the thermal reaction of cobalt(II) acetate with pivalic acid to give the clusters [\({\text{Co}}_{6}^{{{\text{III}}}}\)(μ4-O)2(μ3-OH)2(OH)2(μ-OOCtBu)9]+(OOCtBu)–(HOOCtBu) and \({\text{Co}}_{{14}}^{{{\text{III}}}}\)(μ5-O)2(μ3-O)2(μ3-OH)12(OH)4(μ-OOCtBu)8-(OOCtBu)10⋅ 2[OC(=O)C3H6][OC(H)(OH)C3H6] [3, 4]. The crystal cell of the latter compound contains two butyrolactone and two 2-hydroxytetrahydrofuran solvate molecules, resulting from decomposition of 2-hydroperoxytetrahydrofuran, which is formed upon conjugate reaction of air oxygen with THF in the presence of Co(II) atoms.
Similar polynuclear manganese carboxylates and oxo and hydroxo carboxylates are well known (by November, 2020, the Cambridge Crystallographic Data Centre contained approximately 7000 structurally characterized manganese carboxylate compounds [5]). The obvious interest in these complexes is related to their use in many fundamental fields of modern chemistry, including single-molecule magnets (preparation of polynuclear complexes and clusters with high-spin metal atoms [6–15]), catalysis [16–19], bioinorganic chemistry (modeling of the active part of natural enzymes [20–26]), and so on.
Previously, we showed that the reaction of manganese(II) acetate hydrate with cymantrenecarboxylic acid in methanol results in the formation of the complex Mn[OOCC5H4Mn(CO)3]2[O(H)Me]4 [27]. In this communication, we report the structure of products of a similar reaction, but carried out in THF.
EXPERIMENTAL
Commercial Mn2(OOCMe)4(OH2)4 (Acros) was used; cymantrenecarboxylic acid was synthesized by the procedure reported in [28].
Synthesis of the complex [Mn(Thf)2(OH2)4][OOCC5H4Mn(CO)3]2 (I) and the cluster Mn6(µ4-O)2[µ,η2-OOCC5H4Mn(CO)3]2[µ-OOCC5H4Mn(CO)3]8(OH2)4· 5C6H6·THF·3H2O (II). A solution of HOOCC5H4Mn(CO)3 (0.4 g, 1.6 mmol) in THF (10 mL) was added to Mn(CH3COO)2⋅4H2O (0.2 g, 0.8 mmol), and the mixture was refluxed in an inert atmosphere (argon) for 2 h. The resulting homogeneous solution was concentrated to ~4 mL and left to cool down to room temperature in an oil bath. The colorless crystals of complex I, highly unstable at room temperature, which were formed within 24 h, were separated from the mother liquor by decanting, washed successively with cold benzene (10 mL) and hexane (10 mL), and dried in an argon flow. After 10–15 min in argon, the crystals decomposed being converted to a powder. In air, the mother liquor rapidly (after ~30 min) changed its color. Benzene (5 mL) was added to the resulting brown solution, and the mixture was left overnight under an exhaust hood in an open flask. The resulting brown crystals of cluster II were separated from the solution by decanting, washed successively with cold benzene (10 mL) and hexane (10 mL), and dried in an argon flow. The single crystals of II also proved to be unstable at room temperature.
The yield of I was 0.09 g (15%).
For C26H32O16Mn3 Anal calcd., % | C, 40.80 | H, 4.21 |
For C26H32O16Mn3–THF C22H24Mn3O15 Anal. calcd., % | C, 38.11 | H, 3.49 |
For C26H32O16Mn3–2 THF C18H16O14Mn3 Anal. calcd., % | C, 34.81 | H, 2.59 |
Found (powder), % | C, 38.12 | H, 2.87 |
Since compound I was unstable and elemental analysis was performed for a powder, the calculated and experimental data were in poor agreement. However, according to calculations, some of coordinated THF seems to be evaporated during decomposition of the complex (this is reflected in the results of elemental analysis).
IR (powder): 2016 s, 1911 s, 1682 w, 1567 m, 1480 s, 1388 s, 1363 s, 1200 m, 1015 m, 924 w, 837 w, 796 m, 663 s, 627 s, 535 s, 489 m, 465 m, 440 w, 414 w, 405 w.
The yield of II was 0.25 g (38%).
For C90H48O56Mn16 Anal. calcd., % | C, 37.22 | H, 1.66 |
For C90H54O59Mn16 (C90H48Mn16O56·3H2O) Anal. calcd., % | C, 36.54 | H, 1.84 |
Found (powder), % | C, 36.18 | H, 2.04 |
IR (powder): 2018 s, 1919 s, 1539 m, 1481 s, 1391 s, 1361 s, 1260 w, 1199 w, 1029 m, 926 w, 838 w, 790 m, 665 s, 628 s, 538 s, 489 m, 471 m, 453 m, 433 w, 418 w.
Due to the extremely low stability of compounds I and II, no satisfactory results of chemical analysis could be obtained.
The IR spectra of the compounds were measured on a Perkin-Elmer Spectrum 65 Fourier Transform IR spectrometer in the attenuated total reflectance (ATR) mode in the frequency range of 400–4000 cm–1.
The single crystals for X-ray diffraction were taken out directly from the mother liquor and rapidly transferred into the flow of evaporating liquid nitrogen.
X-ray diffraction study of I and II was carried out by the standard procedure on a Bruker SMART Apex II automated diffractometer equipped with a CCD array detector (MoKα radiation, λ = 0.71073 Å, graphite monochromator, ω-scan mode). The structures were refined using the SHELXTL PLUS program package (PC version) [29–32]. The structures were solved by direct methods and refined by the least squares method in the anisotropic approximation for all non-hydrogen atoms. The positions of hydrogen atoms of the coordinated water molecules in I were derived from difference Fourier maps and refined isotropically, and the other hydrogen atoms of I and II were located geometrically and refined in the riding model.
The crystallographic data and structure refinement details for I and II are summarized in Table 1, and bond lengths and bond angles are in Tables 2 and 3, respectively.
The structural data were deposited with the Crystallographic Data Centre (CCDC nos. 2059074 (I) and 2059075 (II); http://www.ccdc.cam.ac.uk/).
RESULTS AND DISCUSSION
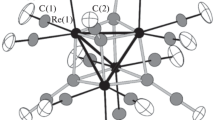
It was found that, unlike the reaction of manganese(II) acetate hydrate with cymantrenecarboxylic acid in methanol, this reaction in donor polar THF affords extremely unstable colorless complex [Mn(OH2)4(Thf)2][OOCC5H4Mn(CO)3]2 (I, 15% yield). According to X-ray diffraction data, the metal atom in the centrosymmetric mononuclear complex I (Tables 1, 2, Fig. 1) has a distorted octahedral environment composed of four oxygen atoms of equatorially coordinated water molecules (Mn(1)–O, 2.066(3)–2.089(3) Å) and two oxygen atoms of axially coordinated THF molecules (Mn(1)–O, 2.130(4) Å). The oxygen atoms of two outer-sphere cymantrenecarboxylate anions form short bonds with water hydrogen atoms, thus giving a 2D polymer (Fig. 2) (O(4)…O(2) (O(2A), 2.658 Å (2.714 Å)); O(5)…O(3) (O(3A), 2.671 Å (2.714 Å)). Note that all cyclopentadienyl moieties in the polymer are parallel.
Previously, it was found that a similar reaction carried out in THF on heating to 50°C, with addition of hexane and keeping of the obtained solution in a refrigerator at 5°C results in the formation of crystals of the heterocarboxylate 1D-polymer {Mn2[µ-OOCC5H4Mn(CO)3]2(µ-OOCMe)(µ-OOCC5H4Mn(CO)3)(Thf)2}n (A) [33]. Presumably, the following main equilibria may occur in the reaction mixture in polar THF at a temperature where there is no obvious removal of one of the reactants: Mn(OOCMe)2(OH2)4 + HOOCC5H4Mn(CO)3 ↔ (I) + 2HOOCMe ↔ (A) + HOOCMe + 4H2O + HOOCC5H4Mn(CO)3 ↔ Mn[OOCC5H4Mn(CO)3]2(Thf)4 (B) + 2HOOCMe + 4H2O.
It cannot be ruled out that the formation of single crystals of one of the complexes present in the solution is determined by the crystallization conditions, but the presumed adduct B has not yet been isolated in the single crystalline state.
It is noteworthy that similar equilibria occur in the solution formed upon the reaction of manganese acetate hydrate with benzoic acid in hot toluene, resulting in the formation of the polymer {Mn5-(OO-CMe)6(OOCPh)4}n [34], and the reaction of Zn(OOCMe)2(OH2)2 with cymantrenecarboxylic acid in acetonitrile at 50°C, giving 1D polymer {Zn[OOCC5H4Mn(CO)3](µ-OOCMe)(OH2)}n [35].
In the presence of air oxygen, the colorless mother liquor of this reaction rapidly turns brown, and addition of benzene induces crystallization of the hexanuclear cluster Mn6(µ4-O)2(µ,η2-OOCC5H4Mn(CO)3)2-(µ-OOCC5H4Mn(CO)3)8(OH2)4·5C6H6·THF·3H2O (II, 35% yield).
According to X-ray diffraction data, six manganese atoms in II (Table 3, Figs. 3, 4) are connected by two tetradentate bridging oxygen atoms (O(1)–Mn(1), 2.180(6); O(1)–Mn(2), 1.896(6); O(1)–Mn(3), 1.869(6); O(1)–Mn(6), 2.209(6) Å; O(2)–Mn(2), 1.873(6); O(2)–Mn(3), 1.910(6); O(2)–Mn(4), 2.177(7); O(2)–Mn(5), 2.192(6) Å). The distribution of M–O bond lengths suggests that the Mn(2) and Mn(3) atoms are in the +3 oxidation state (stronger Lewis acids), while the oxidation state of the other metal atoms is +2. This assumption is confirmed by the considerable difference between the “metal–bridging anion oxygen” bond lengths (MnII(1)–O, 2.113(7)–2.339(7); MnIII(2)–O, 1.954(7)–2.235(7); MnIII(3)–O, 1.957(7)–2.235(7); MnII(4)–O, 2.120(8)– 2.362(7); MnII(5)–O, 2.158(7)–2.278(7); MnII(6)–O, 2.110(7)–2.271(7) Å) and, hence, M…M distances (MnIII(2)…MnIII(3), 2.8220(17); MnIII(2),(3)…MnII, 3.160(2)–3.502(3); MnII…MnII, 3.781(3)–4.709(3) Å).
Finally, each MnIII atom has a distorted octahedral environment composed of the oxygen atoms of the bridging anions, while the environment of MnII atoms is completed by the oxygen atoms of coordinated water molecules (Mn–O, 2.215(8)–2.230(8) Å) (Table 3, Fig. 4).
It is worth noting that three identified solvate water molecules are hydrogen-bonded to one another and to oxygen atoms of coordinated H2O and the bridging anion, while the other solvate molecules have no noticeable contacts with the atoms of cluster II.
The hexanuclear mixed-valence manganese carboxylates \({\text{Mn}}_{4}^{{{\text{II}}}}{{{\text{L}}}_{4}}\)(µ4-O)2\({\text{Mn}}_{2}^{{{\text{III}}}}\)(OOCR)10 (L = manganese(II)-coordinated two-electron donor) are well known [36]; they have been prepared by a variety of methods: reactions of manganese(II) salts (chlorides, carbonates) with sodium or potassium carboxylates; ligand exchange of anions in the manganese acetate with anions of the acid followed by oxidation with air oxygen in polar solvents (H2O, MeCN, THF), hydrogen peroxide, or manganese compounds with high oxidation states (MnO\(_{4}^{-}\)) [37–52].
Thus, this study demonstrated that, unlike the reaction of manganese(II) acetate hydrate with cymantrenecarboxylic acid in methanol, which gives the adduct Mn[OOCC5H4Mn(CO)3]2[O(H)Me]4, stable to air oxygen, with four coordinated methanol molecules, a similar reaction in THF affords the mononuclear complex [Mn(OH2)4(Thf)2][OOCC5H4Mn(CO)3]2, in which the anions occupy the outer-sphere positions. This complex is readily oxidized in THF in air, giving rise to a hexanuclear cluster containing manganese atoms in different oxidation states. Note that the metal atoms located in the organometallic part of the molecule are not oxidized.
REFERENCES
Karnojitzki, V., Les Peroxydes Organiques, Hermann, Paris, 1958.
Hawkings, E.G.E., Organic Peroxides. Their Formation and Reactions, London: Spon, 1961.
Nefedov, S.E., Uvarova, M.A., Golubichnaya, M.A., et al., Russ. J. Coord. Chem., 2014, vol. 40, p. 358.
Nefedov, S.E. and Denisova, T.O., Russ. J. Inorg. Chem., 2006, vol. 51, p. 1404. https://doi.org/10.1134/S0036023606090063
CSD. Version 5.42 (November 2020).
Friedman, J.R. and Sarachik, M.P., Annu. Rev. Cond. Mat., 2010, no. 1, p. 109. https://doi.org/10.1146/annurev-conmatphys-070909-104053
Kittilstved, K.R., Liu, W.K., and Gamelin, D.R., Nat. Mater., 2006, vol. 5, p. 291. https://doi.org/10.1038/nmat1616
Wernsdorfer, W., Aliaga-Alcalde, N., Hendrickson, D.N., and Christou, G., Nature, 2002, vol. 416, p. 406. https://doi.org/10.1038/416406a
Martin, J.D. and Hess, R.F., Chem. Commun., 1996, vol. 288, p. 2419. https://doi.org/10.1039/CC9960002419
Liu, S., Bremer, M.T., Lovaasen, J., et al., Inorg. Chem., 2008, vol. 47, p. 1568. https://doi.org/10.1021/ic7020879
Geier, S., Mason, J.A., Bloch, E., et al., Chem. Sci., 2013, p. 2054. https://doi.org/10.1039/C3SC00032J
Aromí, G. and Brechin, E.K., in Structure and Bonding: Single-Molecule Magnets and Related Phenomena, Winpenny, R., Ed., Berlin: Springer, 2006, vol. 122, p. 1.
Inglis, R., White, F., Piligkos, S., et al., Chem. Commun., 2011, vol. 47, p. 3090. https://doi.org/10.1039/c0cc05750a
Abasi, P., Quinn, K., Alexandropoulos, D.I., et al., J. Am. Chem. Soc., 2017, vol. 139, p. 15644. https://doi.org/10.1021/jacs.7b10130
Mishra, A., Wernsdorfer, W., Abboud, K.A., and Christou, G., Inorg. Chem., 2006, vol. 45, p. 10197. https://doi.org/10.1021/ic061334d
de Boer, J.W., Brinksma, J., Browne, W.R., et al., J. Am. Chem. Soc., 2005, vol. 127, p. 7990. https://doi.org/10.1021/ja050990u
Talsi, E.P. and Bryakov, K.P., Coord. Chem. Rev., 2012, vol. 256, nos. 13–14, p. 1418. https://doi.org/10.1016/j.ccr.2012.04.005
Castaman, S.T., Nakagaki, Sh., Ribeiro, R.R., et al., J. Mol. Catal. A, 2009, vol. 300, nos. 1–2, p. 89. https://doi.org/10.1016/j.molcata.2008.10.037
Waiba, S. and Maji, B., ChemCatChem, 2020, vol. 12, p. 1891. https://doi.org/10.1002/cctc.201902180
Lippard, S.J. and Berg, J.M., Principles of Bioinorganic Chemistry, Mill Valley: Univ. Science Books, 1994, p. 199.
Kanady, J.S., Tsui, E.Y., Day, M.W., and Agapie, T., Science, 2011, vol. 333, p. 733. https://doi.org/10.1126/science.1206036
Larson, V.A., Battistella, B., Ray, K., et al., Nat. Rev. Chem., 2020, vol. 4, p. 404. https://doi.org/10.1038/s41570-020-0197-9
Tsui, E.Y., Kanady, J.S., and Agapie, T., Inorg. Chem., 2013, vol. 52, no. 24, p. 13833. https://doi.org/10.1021/ic402236f
Lee, H.B., Marchiori, D.A., Chatterjee, R., et al., J. Am. Chem. Soc., 2020, vol. 142, no. 8, p. 3753. https://doi.org/10.1021/jacs.9b1037
Reed, Ch.J. and Agapie, T., J. Am. Chem. Soc., 2018, vol. 140, no. 34, p. 10900. https://doi.org/10.1021/jacs.8b06426
Beal, N.J. and O’Malley, P.J., J. Am. Chem. Soc., 2016, vol. 138, no. 13, p. 4358. https://doi.org/10.1021/jacs.6b02600
Uvarova, M.A. and Nefedov, S.E., Russ. J. Inorg. Chem., 2015, p. 1074. https://doi.org/10.1134/S003602361509020X
Nesmeyanov, A.N., Anisimov, K.N., Kolobova, N.E., and Makarov, Y.V., Bull. USSR Acad. Sci., Div. Chem. Sci., 1968, p. 672.
SMART (control) and SAINT (integration) Software. Version 5.0, Madison: Bruker AXS Inc., 1997.
SAINT. Area-Detector Integration Sofware, Madison: Bruker AXS Inc., 2012.
Sheldrick, G.M., SADABS. Program for Scaling and Correction of Area Detector Data, Göttingen: Univ. of Göttingen, 1997.
Sheldrick, G.M., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053229614024218
Uvarova, M.A. and Nefedov, S.E., Russ. J. Inorg. Chem., 2015, vol. 60, no. 11, p. 1348. https://doi.org/10.1134/S0036023615110212
Liu, Sh., Bremer, M.T., Lovaasen, J., et al., Inorg. Chem., 2008, vol. 47, p. 1568. https://doi.org/10.1021/ic7020879
Uvarova, M.A., Ageshina, A.A., Grineva, A.A., et al., Russ. J. Inorg. Chem., 2015, vol. 60, no. 11, p. 566.
Baca, S.G., Advances in Chemistry Research, Taylor, J.C., Ed., New York: Nova Science, 2018, vol. 43, p. 81.
Wemple, M.W., Tsai, H.-L., Wang, S., et al., Inorg. Chem., 1996, vol. 35, p. 6437. https://doi.org/10.1021/ic9603013
Malaestean, I.L., Ellern, A., Leusen, J., et al., Cryst-EngComm, 2014, vol. 1, p. 6523. https://doi.org/10.1039/C4CE00504J
Kornowicz, A., Komorski, S., Wrobel, Z., et al., Dalton Trans., 2014, vol. 43, p. 3048. https://doi.org/10.1039/C3DT53211A
Halcrow, M.A., Streib, W.E., Folting, K., and Christou, G., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1995, vol. 51, p. 1263. https://doi.org/10.1107/S0108270194013764
Kushch, L.A., Shilov, G.V., Morgunov, R.B., and Yagubskii, E.B., Mendeleev Commun., 2009, vol. 19, p. 170. https://doi.org/10.1016/j.mencom.2009.05.021
Batsanov, A.S., Struchkov, Yu.T., Timco, G.A., et al., Russ. J. Coord. Chem., 1994, vol. 20, p. 604.
Malaestean, I.L., Kravtsov, V.Ch., Speldrich, M., et al., Inorg. Chem., 2010, vol. 49, p. 7764. https://doi.org/10.1021/ic100541m
Schake, A.R., Vincent, J.B., Li Qiaoying, et al., Inorg. Chem., 1989, vol. 28, p. 1915.
Karsten, P. and Strähle, J., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1998, vol. 54, p. 1403. https://doi.org/10.1107/S0108270198005861
Kohler, K., Roesky, H.W., Noltemeyer, M., et al., Chem. Ber., 1993, vol. 126, p. 921. https://doi.org/10.1002/cber.19931260411
Stamatatos, T.C., Foguet-Albiol, D., Perlepes, S.P., et al., Polyhedron, 2006, vol. 25, p. 1737. https://doi.org/10.1016/j.poly.2005.11.019
Kiskin, M.A., Sidorov, A.A., Fomina, I.G., et al., Inorg. Chem. Commun., 2005, vol. 8, p. 524. https://doi.org/10.1016/j.inoche.2005.03.005
Kar, P., Haldar, R., Gomez-Garcia, C.J., and Ghosh, A., Inorg. Chem., 2012, vol. 51, p. 4265. https://doi.org/10.1021/ic2027362
Gerbier, P., Ruiz-Molina, D., Gomez, J., et al., Polyhedron, 2003, vol. 22, p. 1951. https://doi.org/10.1016/S0277-5387(03)00158-X
Darii, M., Filippova, I., Hauser, J., et al., Crystals, 2018, vol. 8, no. 2, p. 100. https://doi.org/10.3390/cryst8020100
Ovcharenko, V., Fursova, E., Romanenko, G., and Ikorskii, V., Inorg. Chem., 2004, vol. 43, p. 3332. https://doi.org/10.1021/ic049859y
ACKNOWLEDGMENTS
X-ray diffraction analysis and IR spectroscopic studies were performed using equipment of the Center for Collective Use of Physical Methods of Investigation, Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, which is supported by the state assignment for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, in the field of fundamental research.
Funding
This study was performed within the state assignment for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, in the field of fundamental research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Uvarova, M.A., Nefedov, S.E. Formation of a Cluster with the \({\text{M}}_{4}^{{{\text{II}}}}{\text{M}}_{2}^{{{\text{III}}}}\) Metal Core upon the Oxidation of Manganese(II) Cymantrenecarboxylate Adduct with Air Oxygen in Tetrahydrofuran. Russ J Coord Chem 47, 760–768 (2021). https://doi.org/10.1134/S1070328421110051
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328421110051