Abstract
The reaction of (η5-C5H5)Ni(SIMes)Cl with n-propylmercaptane and NEt3 in CH2Cl2 affords the thiolate carbene nickel complex (η5-C5H5)Ni(SIMes)SnPr (I) (SIMes is 1,3-dimesitylimidazol-2-ylidene), which reacts with W(CO)5(THF) to form the heterometallic complex (η5-C5H5)Ni(SIMes)(μ2-SnPr)W(CO)5 (II). The reaction of complex I with (η5-С5H5)Mn(CO)2(THF) affords compound (η5-C5H5)Ni(SIMes)(μ2-SnPr)(η5-С5H5)Mn(CO)2 (III). The structures of compounds I, II, and III are determined by X-ray structure analysis (CIF file CCDC nos. 2024873 (I), 2024874 (II), and 2024875 (III)). According to the data of thermogravimetry and differential scanning calorimetry, the thermal decomposition of complexes II and III occurs stepwise in ranges of 101–500 and 119–550°С, and no ligand elimination is observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
N-Heterocyclic carbenes (NHCs) based on imidazol-2-ylidene are the ligands with simultaneously strong σ-donor and weak π-acceptor properties, which makes it possible to use them as stabilizing ligands in the chemistry of organoelement compounds [1, 2]. On the one hand, these ligands can stabilize the nickel complexes in different oxidation states [3, 4]. On the other hand, owing to the variation of the substituents at the nitrogen atoms, they can form a sufficient shielding of the metal center thus providing a high catalytic activity of compounds of this type. We have previously shown [5, 6] the possibility of synthesizing the heterometallic nickel complexes with 1,3-dimethylimidazol-2-ylidene (MeIm). The thiolate complex of cyclopentadienylnickel with 1,3-dimesitylimidazol-2-ylidene (SIMes), (η5-C5H5)Ni-(SIMes)-SnPr (I), is used as the metal ligand for the synthesis of the heterometallic derivatives.
EXPERIMENTAL
All procedures associated with the synthesis and isolation of the compounds were carried out under argon and in dehydrated solvents. Compound (η5‑C5H5)Ni(SIMes)Cl was synthesized using a known procedure [7]. Commercial nickelocene (99%, OOO Dalkhim), 1,3-bis(2,4,6-trimethylphenyl)imidazolium chloride (95+%, OOO Dalkhim), and W(CO)6 (97%, Sigma-Aldrich) were used as received. Triethylamine (≥99.5%, Sigma-Aldrich) was dried over molecular sieves 3Е Sigma-Aldrich. UV irradiation was carried out using a DRL 250V lamp without a glass flask covered with the luminophore. Chemical analyses were carried out on an EA3000 CHNS analyzer (Euro Vector). A Bruker Alpha FT-IR spectrometer with a Platinum ATR accessory for operating in the attenuated total reflectance mode was used for IR spectroscopy. 1H (300.13 MHz) and 13C (75.4 MHz) NMR spectra were recorded on a Bruker AV 300 spectrometer, and chemical shifts are presented relative to tetramethylsilane. Simultaneous (TG–DSC) thermal analysis (TG–DSC is thermogravimetry coupled with differential scanning calorimetry) of the synthesized complexes was carried out using an SDT Q-600 thermoanalyzer. Controlled heating was conducted in Al2O3 crucibles in a range of 20–600°С with a rate of 10°/min in an argon flow (flow rate 250 mL/min), and the weight of the starting sample was varied in a range of 3.5–10.5 mg.
Synthesis of (η5-C5H5)Ni(SIMes)(SnPr) (I). n‑Propylmercaptane (0.1 mL, 1.105 mmol) and triethylamine (0.12 mL, 0.865 mmol) were added to a pink solution of (η5-C5H5)Ni(SIMes)Cl (0.301 g, 0.649 mmol) in toluene (15 mL). The reaction mixture was stirred for 48 h. The resulting brown solution was filtered, and the solvents were removed under reduced pressure. The obtained brown oily residue was crystallized from hexane at –24°С. The yield of the green-brown crystals was 0.282 g (87%).
For C29H36N2SNi (FW = 503) | |||
Anal. calcd., % | C, 69.20 | H, 7.21 | N, 5.56 |
Found, % | C, 69.32 | H, 7.32 | N, 5.47 |
IR (ν, cm–1): 3170 w, 3135 w, 3102 w, 3018 w, 2951 m.br, 2913 m.br, 2862 w, 2194 w, 1718 w.br, 1651 w, 1607 w, 1548 w, 1520 w, 1483 m.br, 1459 m.br, 1396 s, 1372 m, 1350 m, 1322 s, 1281 w, 1264 s, 1246 m, 1218 m, 1161 w, 1113 w, 1082 m, 1054 m, 1031 m, 1015 s, 985 m, 960 m, 923 s, 890 m, 848 s, 834 m, 824 m, 775 vs, 741 m, 725 vs, 692 vs, 645 m, 593 m, 575 m, 501 w, 452 w, 430 m. 1H NMR (C6D6), δ, ppm: 1.01 (t, 3J = 7.11 Hz, 3H, Me, Pr), 1.53–1.77 (m, 4H, 2CH2, Pr), 2.15 (s, 6H, 2Me, Mes), 2.22 (s, 4Me, Mes), 4.92 (s, 5H, Cp), 6.17 (s, 2H, 2CH, Im), 6.85 (s, 4H, 2CH, Mes). 13C{H} NMR (C6D6), δ, ppm: 15.0 (Me, Pr), 19.3 Me, Mes), 21.4 (Me, Mes), 28.8 (CH2, Pr), 34.9, (CH2, Pr), 91.7 (Ср), 123.8 (CH, Im), 129.6 (Mes), 136.6 (Mes), 138.0 (Mes), 139.0 (Mes), 174.8 (C, Im).
Synthesis of (η5-C5H5)Ni(SIMes)(μ2-SnPr)W-(CO)5 (II). n-Propylmercaptane (0.08 mL) and triethylamine (0.09 mL) were added to a pink solution of (η5-C5H5)Ni(SIMes)Cl (0.201 g, 0.433 mmol) in toluene (10 mL). The reaction mixture was stirred for 48 h. The resulting brown solution was filtered, and the solvents were removed under reduced pressure. A yellow solution obtained by the UV irradiation of W(CO)6 (0.152 g) in THF (20 mL) for 30 min with water cooling was added to the brown oily residue. The solvent was removed under reduced pressure, and the residue was extracted with boiling hexane. Brown crystals were formed on storage at –24°С for 18 h and recrystallized from a toluene–hexane (1 : 1) mixture. The yield was 0.179 g (49%).
IR (ν, cm–1): 2925 vw, 2057 vw, 1962 vw, 1904 vs, 1874 m, 1486 vw.br, 1400 vw, 1377 vw, 1319 vw, 1266 vw.br, 1034 vw.br, 925 vw, 853 vw, 785 vw, 734 vw, 698 vw, 678 vw, 599 vw, 584 vw.br. 1H NMR (C6D6), δ, ppm: 0.70 (t, 3J = 7.07 Hz, 3H, Me, Pr), 1.17–1.37 (m, 2H, CH2, Pr), 1.58–1.74 (m, 2H, CH2, Pr), 2.03 (s, 12H, 4Me, Mes), 2.13 (s, 6H, 2Me, Mes), 4.89 (s, 5H, Cp), 5.99 (s, 2H, 2CH, Im), 6.78 (s, 4H, CH, Mes). 13C{H} NMR (C6D6), δ, ppm: 14.3 (Me, Pr), 19.1 Me, Mes), 21.4 (Me, Mes), 26.9 (CH2, Pr), 44.4 (CH2, Pr), 93.4 (Ср), 125.0 (CH, Im), 130.0 (Mes), 135.9 (Mes), 137.3 (Mes), 139.4 (Mes), 168.1 (C, Im), 201.1 (CO), 201.5 (CO).
For C34H36N2O5SNiW (FW = 827) | |||
Anal. calcd., % | C, 49.36 | H, 4.39 | N, 3.38 |
Found, % | C, 49.78 | H, 5.43 | N, 3.38 |
Synthesis of (η5-C5H5)Ni(SIMes)(μ2-SnPr)(η5-С5H5)Mn(CO)2 (III). A red solution obtained by the UV irradiation of C5H5Mn(CO)3 (0.06 g, 0.24 mmol) in THF (15 mL) at –40°С for 50 min was added to Cp(Mes2Im)SNiPr (0.12 g, 0.24 mmol). The solvent was removed under reduced pressure. The resulting dark green oily residue was triturated with hexane (30 mL), dried in vacuo, and crystallized from a toluene–hexane (1 : 1) mixture at –24°С for 18 h. The yield of the dark green crystals was 0.085 g (52%).
IR (ν, cm–1): 3129 vw.br, 2958 w.br, 2919 vw.br, 2866 vw, 1896 vs, 1827 vs.br, 1608 vw, 1567 vw, 1485 w.br, 1461 w.br, 1422 w, 1397 w, 1375 w, 1355 w, 1318 m, 1264 m, 1224 w, 1163 vw, 1112 vw, 1081 w, 1013 w.br, 974 vw, 960 vw, 924 w, 894 vw, 852 w, 836 w, 785 m, 735 m, 697 m, 660 w, 611 m, 586 m, 525 w, 477 w, 432 vw. 1H NMR (C6D6), δ: 0.80 (t, 3J = 7.06 Hz, 3H, Me, Pr), 1.25 (m, 2H, CH2, Pr), 1.56 (m, 2H, CH2, Pr), 2.09 (s, 12H, Me, Mes), 2.17 (s, 6H, Me, Mes), 4.36 (s, 5H, Cp), 5.05 (s, 5H, Cp), 6.09 (s, 2H, 2CH, Mes2Im), 6.85 (s, 4H, CH, Mes). 13C{H} NMR (C6D6), δ, ppm: 14.8 (Me, Pr), 19.3 Me, Mes), 21.5 (Me, Mes), 26.3 (CH2, Pr), 45.4 (CH2, Pr), 83.2 (Cp), 93.2 (Ср), 124.3 (CH, Im), 130.0 (Mes), 136.0 (Mes), 137.5 (Mes), 139.3 (Mes), 172.8 (C, Im), 238.1 (CO).
For C36H41MnN2NiO2S (FW = 679) | |||
Anal. calcd., % | C, 63.64 | H, 6.08 | N, 4.12 |
Found, % | C, 63.58 | H, 6.21 | N, 3.98 |
X-ray structure analysis (XSA). Diffraction data for compounds I and II were obtained at 100 K and those for compound III were obtained at 240 K on a Bruker SMART APEX II diffractometer equipped with a Photon two-coordinate detector (graphite monochromator, λ(MoKα) = 0.71073 Å, ω scan mode). An absorption correction was applied by the method of multiple measurements of equivalent reflections using the SADABS program [8]. The crystal structures were solved using the SHELXT program and refined by full-matrix least squares in the anisotropic (except for hydrogen atoms) approximation using the SHELXL [9] and OLEX2 [10] programs. The positions of the hydrogen atoms of the organic ligands were calculated geometrically and refined by the riding model. The crystallographic data and details of diffraction experiments are presented in Table 1.
The full tables of interatomic distances and bond angles, coordinates of atoms, and atomic shift parameters were deposited with the Cambridge Crystallographic Data Centre (CIF file CCDC nos. 2024873 (I), 2024874 (II), and 2024875 (III); https://www. ccdc.cam.ac.uk/structures/).
RESULTS AND DISCUSSION
Thiolate complexes based on cyclopentadienylnickel containing sterically loaded N-heterocyclic carbene ligands can be synthesized either by the treatment of the 17-electron complex (η5-C5H5)Ni(NHC) with diaryl sulfide [11], or by the synthetically simpler substitution of halogen by SR in (η5-C5H5)-Ni(NHC)Cl [12].
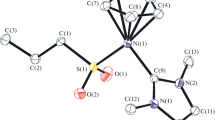
The use of the second approach made it possible to synthesize the (η5-C5H5)Ni(SIMes)SnPr complex (I) by the reaction of (η5-C5H5)Ni(SIMes)Cl with n-propylmercaptane and triethylamine. The single crystals of complex I were obtained by vapor diffusion. According to the XSA data (Fig. 1), the Ni–S and Ni–C bond lengths in compound I are 2.1873(5) and 1.874(2) Å, respectively, which are lower than the sum of covalent radii (SCR): rNi + rS = 2.29 and rNi + rC = 1.97 Å [13].
The reaction of complex I with the W(CO)5(THF) adduct, which was synthesized by the UV irradiation of a solution of W(CO)6 in THF, affords the (η5‑C5H5)Ni(SIMes)(μ2-SnPr)W(CO)5 complex (II) bearing the bridging thiolate ligand.
The IR spectrum of complex II exhibits bands at 2057, 1962, 1904, and 1874 cm–1 corresponding to stretching vibrations of the carbonyl groups of the W(CO)5 fragment. According to the XSA data (Fig. 2), the Ni–S bond lengths in complex II (2.2139(8) and 2.2066(8) Å) are lower than the SCR (SCR = rNi + rS = 2.29 Å [13]) but higher than a similar bond length in complex I and in the (η5-C5H5)Ni(MeIm)(μ2-SnPr)W(CO)5 complex (IIа) with a similar structure (2.1933(7) Å) [5]. The Ni–С bonds with the carbene ligand are 1.903(3) and 1.900(3) Å, which are lower than the corresponding SCR (rNi + rC = 1.97 Å) but higher than those in complex IIa (1.882(3) and 1.879(3) Å). This indicates a higher donor ability of the MeIm fragment compared to SIMes. The W–S bond lengths (2.5800(7) and 2.5931(7) Å) are lower than the corresponding SCR (RNi + RC = 1.97 Å, RW + RS = 2.67 Å [13]) and comparable with those for complex IIa. In complex II, the NiSW angles are 116.46(3)° and 117.30(3)°. An increase in these values over those in complex IIa (109.66(3)° and 112.69(3)°) can be explained by the stronger steric repulsion of N-heterocyclic carbene and carbonyl groups of the W(CO)5 fragment compared to complex Ia.
Molecular structure of complex II (thermal ellipsoids of atoms of 50% probability). Hydrogen atoms are omitted for clarity. One of two independent molecules is shown. Selected bond lengths and bond angles: W(1)–S(1) 2.5931(7), W(1)–C(2) 2.045(3), W(1)–C(4) 2.036(3), W(1)–C(3) 2.048(4), W(1)–C(1) 1.975(3), W(1)–C(5) 2.035(4), Ni(1)–S(1) 2.2139(8), and Ni(1)–C(6) 1.903(3) Å and Ni(1)S(1)W(1) 116.46(3)°.
The reaction of complex I with the adduct (η5‑С5H5)Mn(CO)2(THF), which was synthesized by the UV irradiation of a solution of (η5-С5H5)-Mn(CO)3 in THF, affords the heterometallic complex (η5-C5H5)Ni(SIMes)(μ2-SnPr)(η5-С5H5)Mn(CO)2 (III).
The IR spectrum of complex III exhibits bands at 1896 and 1827 cm–1 corresponding to stretching vibrations of the carbonyl groups of the (η5-С5H5)-Mn(CO)2 fragment. The corresponding frequencies for the close in structure (η5-C5H5)Ni(MeIm)(μ2-SnPr)Mn(CO)2(η5-C5H5) complex (IIIa) are 1891 and 1803 cm–1 [6]. According to the XSA data (Fig. 3), the Ni–S, Ni–C, and Mn–S bond lengths (2.2153(7), 1.898(3), and 2.3538(8) Å) are higher than the corresponding values in complex IIa (2.2087, 1.884, and 2.3308 Å) but lower than the SCR (RNi + RS = 2.29 Å, RNi + RC = 1.97 Å, RMn + RS = 2.44 Å).
Molecular structure of complex III (thermal ellipsoids of atoms of 30% probability). Hydrogen atoms are omitted for clarity. One of two independent molecules is shown. Selected bond lengths and bond angles: Ni(1)‒S(1) 2.2153(7), Ni(1)–C(1) 1.898(3), Mn(1)–S(1) 2.3538(8), and S(1)–C(27) 1.828(3) Å and Ni(1)S-(1)Mn(1) 122.11(3)°.
According to the TG–DSC data, the thermal decomposition of complex II is stepwise: one CO molecule is lost at 101–137°С, more two CO molecules are lost in a range of 137–156°С, the remained two CO molecules are eliminated in a range of 156–190°С, and other organic ligands are eliminated partially without pronounced boundaries to 500°С. The further heating to 550°С does not result in an additional mass loss. The residue (47.21%) can consist of nickel and tungsten sulfides (NiWS, 33.19%) contaminated with the pyrolysis products of the ligands (14.02%).
The thermal decomposition of complex III proceeds stepwise but without distinnct boundaries: the organic ligands are partially eliminated at 119–550°С. The residue (47.21%) can consist of nickel and manganese sulfides (NiMnS, 21.44%) contaminated with the pyrolysis products of the ligands (13.48%).
Complex IIa is characterized by the complete stepwise elimination of the ligands (at 92–182°С, five СO and C3H7; at 183–550°С, C5H5 and Me2Im) to form a residue of NiWS (exp. 45.95%, theor. 44.35%).
Thus, 1,3-dimethylimidazol-2-ylidene is a more convenient ligand for the synthesis of precursors of inorganic materials than 1,3-dimesitylimidazol-2-ylidene. On the one hand, the present study describes the first examples for the thermal decomposition of the heterometallic complexes with 1,3-dimesitylimidazol-2-ylidene. On the other hand, this study continues the series of works on the isolobal analogy, an original example of which can be the publication of I.B. Sivaev dedicated to memory of A.A. Pasynskii [14].
REFERENCES
Lin, J.C.Y., Huang, R.T.W., Lee, C.S., et al., Chem. Rev., 2009, vol. 109, p. 3561.
Johnson, C. and Albrecht, M., Coord. Chem. Rev., 2017, vol. 352, p. 1.
Danopoulos, A.A., Simler, T., and Braunstein, P., Chem. Rev., 2019, vol. 119, p. 3730.
Lin, C.-Y. and Power, P.P., Chem. Soc. Rev., 2017, vol. 46, p. 5347.
Shapovalov, S.S., Pasynskii, A.A., Skabitskii, I.V., et al., Russ. J. Coord. Chem., 2018, vol. 44, p. 647. https://doi.org/10.1134/s1070328418110076
Shapovalov, S.S., Tikhonova, O.G., Grigor’eva, M.O., et al., Russ. J. Coord. Chem., 2019, vol. 45, p. 706. https://doi.org/10.1134/s1070328419100063
Kelly, R.A., Scott, N.M., Diez-Gonzalez, S., et al., Organometallics, 2005, vol. 24, no. 14.
Sheldrick G.M., SADABS (2016/2), Göttingen: Univ. of Göttingen, 2016.
Sheldrick, G.M., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, p. 3.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., et al., J. Appl. Crystallogr., 2009, vol. 42, p. 339.
Pelties, S., Herrmann, D., de Bruin, B., et al., Chem. Commun., 2014, vol. 50, p. 7014.
Malyshev, D.A., Scott, N.M., Marion, N., et al., Organometallics, 2006, vol. 25, p. 4462.
Cordero, B., Gómez, V., Platero-Prats, A.E., et al., Dalton Trans., 2008, p. 2832.
Sivaev, I.B., Russ. J. Inorg. Chem., 2020, vol. 65, no. 12, p. 1854. https://doi.org/10.1134/S0036023620120165
ACKNOWLEDGMENTS
X-ray structure analyses were carried out using the equipment of the Center for Collective Use of Physical Methods of Investigation at the Kurnakov Institute of General and Inorganic Chemistry (Russian Academy of Sciences) in terms of the state assignment of the Kurnakov Institute of General and Inorganic Chemistry (Russian Academy of Sciences) in the area of basic research.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 19-33-90199 “Aspiranty.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interes-t.
Additional information
Dedicated to blessed memory of Prof. A.A. Pasynskii
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Shapovalov, S.S., Tikhonova, O.G., Skabitskii, I.V. et al. Heterometallic Nickel Complexes with N-Heterocyclic Carbene: Synthesis, Structure, and Thermal Decomposition. Russ J Coord Chem 47, 546–550 (2021). https://doi.org/10.1134/S1070328421080066
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328421080066