Abstract
The heterometallic complex (L1)Ru(κ2-O2CC5H4)Mn(CO)3(O2CC5H4)Mn(CO)3) (I) (L1 is the pivalate ligand) is synthesized by the reaction of the ruthenium(II) complex (L1)Ru(κ2-O2CCMe3)(O2CCMe3) with cymantrenylcarboxylic acid (HO2CC5H4)Mn(CO)3. The consecutive reactions of nickel acetate with cymantrenenecarboxylic acid and diimine 1,4-di-tert-butyl-1,4-diazabutadiene-1,3 affords the complex (L2)Ni(κ2-O2CC5H4Mn(CO)3)2 (II) (L2 = tBu–N=CH–CH=N–tBu). Complexes I and II are identified by the elemental and X-ray diffraction analyses data (СIF files CCDC nos. 2001354 (I) and 2001355 (II)).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The studies of the chemistry of transition metal carboxylates are related to their catalytic [1] and anticorrosion properties [2] and the possibility of coupling with a DNA molecule [3]. The ruthenium carboxylate complexes are characterized by a high catalytic activity in dehydration, С–С cross coupling, and metathesis of olefins [4–9], whereas nickel carboxylates are used more rarely in homogeneous catalysis [10] and more frequently as precursors for heterogeneous catalysis [11–13].
The use of metal-containing carboxylate ligands is one of the methods for the synthesis of heterometallic complexes [14–18]. The nickel complexes based on cymantrenecarboxylic acid with coordinated acetonitrile, methanol [19], and triphenylphosphine [20] have previously been synthesized.
In this work, we propose approaches to the synthesis of metal-containing nickel(II) and ruthenium(II) carboxylates based on cymantrenenecarboxylic acid, which can act as an IR label due to the carbonyl groups at the manganese atom with the sterically loaded ligands: 4-isopropyl-1-methylbenzene (MeC6\({\text{H}}_{{\text{4}}}^{i}\)Pr, L1) and 1,4-di-tert-butyl-1,4-diazabutadiene-1,3 (tBu–N=CH–CH=N–tBu, L2). It is known that the diazadiene derivatives can act as a bridging or redox-active chelating ligand [21]. Diazadiene L2 with tert-butyl substituents as a chelating ligand providing shielding of the metal center was used in this study. Bases or silver salts are not used in the synthesis of the complexes, which makes it possible to avoid the formation of by-products formed upon the destruction of the cymantrenyl fragment [22].
EXPERIMENTAL
All reactions and procedures for the isolation of products were carried out under argon and in absolutized solvents (benzene, methanol, hexane, and toluene). The initial complex (MeC6H4iPr)Ru(OOCtBu)2 and ligand L2 were synthesized using published procedures [9] and [23], respectively. An ЕА3000 CHNS analyzer (EuroVector) was used for chemical analysis. IR spectra were recorded on a Bruker Alpha FTIR spectrometer with a Platinum ATR accessory for recording spectra in the attenuated total internal reflectance (ATR) mode.
Synthesis of (MeC6H4iPr)Ru(κ2-O2CC5H4)Mn(CO)3(O2CC5H4)Mn(CO)3) (I). A solution of (MeC6H4iPr)Ru(OOCtBu)2 (100 mg, 0.23 mmol) and cymantrenecarboxylic acid (113 mg, 0.46 mmol) in benzene (20 mL) was refluxed for 3 h. The solvent was removed in vacuo, and the solid residue was triturated with hexane (10 mL), washed with hexane (3 × 5 mL), and recrystallized from a benzene–heptane (1 : 1) mixture. The obtained yellow powder was filtered off, washed with pentane (2 × 5 mL), and dried in vacuo. The yield of compound I was 107 mg (64%).
For C28H22O10Mn2Ru (FW = 729) | ||
|---|---|---|
Anal. calcd., % | C, 46.10 | H, 3.04 |
Found, % | C, 46.59 | H, 3.15 |
IR (ν, cm–1): 2021 vs, 1929 vs, (CO) 1640 m, 1610 m (OCOаs) 1440 m, 1430 m (OCOs).
Synthesis of (tBu–N=CH–CH=N–tBu)Ni(κ2-O2CC5H4)Mn(CO)3)2 (II). A solution of Ni(O2CCH3)2· 4H2O (200 mg, 0.8 mmol) and cymantrenecarboxylic acid (400 mg, 0.8 mmol) in methanol (20 mL) was refluxed for 2 h. The solvent was removed in vacuo, L2 (190 mg) was added to the solid residue, and the organics was extracted with toluene (30 mL). Hexane (5 mL) was added to the resulting yellow-green solution, and the mixture was kept at –18°С for 4 days. The yield of green crystals of compound II was 215 mg (37%).
IR (ν, cm–1): 2973 w, 2936 w, 2023 vs, 1931 vs, 1674 m, 1626 w, 1550 m, 1478 w, 1463 w, 1369 m, 1224 w, 1038 w, 977 w, 927 w, 879 m, 621 m, 530 w.
For C28H28N2O10Mn2Ni (FW = 721) | ||
|---|---|---|
Anal. calcd., % | C, 46.64 | H, 3.88 |
Found, % | C, 46.79 | H, 3.52 |
X-ray diffraction analysis (XRD) was carried out on a Bruker APEX II CCD diffractometer for complex I and on a Siemens P4 diffractometer for complex II. An absorption correction was applied by multiple measurements of equivalent reflections using the SAD-ABS program [24] for complex I and using the ψ scan method for complex II. The structures of compounds I and II were determined using the SHELXT program [25] and refined by least squares for F 2 in the anisotropic approximation for non-hydrogen atoms using the SHELX-2014 [25] and OLEX2 [26] program packages. The positions of hydrogen atoms were calculated geometrically. The crystallographic data and structure refinement parameters for compounds I and II are presented in Table 1. Selected bond lengths and bond angles are given in the captions to Figs. 1 and 2. The structural contribution of disordered molecules of the solvate solvent in the crystal of compound II was removed using the SQUEEZE procedure implemented in the PLATON program [27].
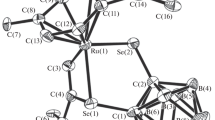
Molecular structure of complex I. Hydrogen atoms are omitted for clarity. Selected bond lengths and bond angles: Ru(1)–O(1) 2.162(2), Ru(1)–O(2) 2.140(2), Ru(1)–O(6) 2.092(2), O(1)–C(1) 1.263(3), O(2)–C(1) 1.274(3), O(6)–C(10) 1.286(3), and O(7)–C(10) 1.226(3) Å, and O(1)C(1)O(2) 118.9(3)° and O(7)C(10)O(6) 126.6(3)°.
Molecular structure of complex II. Hydrogen atoms are omitted for clarity. Selected bond lengths: Ni(1)–N(1) 2.086(3), Ni(1)–N(2) 2.080(3), Ni(1)–O(1) 2.066(3), Ni(1)–O(2) 2.197(3), Ni(1)–O(6) 2.153(3), Ni(1)–O(7) 2.072(3), C(1)–C(2) 1.472(6), C(1)–N(1) 1.265(5), C(2)–N(2) 1.260(5), C(11)–O(1) 1.262(5), C(11)–O(2) 1.251(5), C(20)–O(6) 1.262(5), and C(20)–O(7) 1.263(5) Å.
The coordinates of atoms and other structural parameters were deposited with the Cambridge Crystallographic Data Centre (CIF files CCDC nos. 2001354 (I) and 2001355 (II); http://www. ccdc.cam.ac.uk/data_request/cif).
RESULTS AND DISCUSSION
Complex I was synthesized by the substitution of the pivalate ligands in (MeC6H4iPr)Ru(OOCtBu)2 by cymantrenecarboxylate ligands upon the action of cymantrenecarboxylic acid in boiling benzene.

According to the XRD data, in the structure of complex I, the ruthenium atom is linked with the monodentate and bidentate carboxylate ligands. The Ru–O bond is shorter in the first case (2.092(2) Å), whereas the Ru–O distances for the symmetrically coordinated bidentate (κ2) ligand are 2.162(2) and 2.140(2) Å. The C–O bonds in κ2-carboxylate are also nearly equal (1.263(3) and 1.274(3) Å), whereas for the monodentate ligand the C–O distance with the noncoordinated oxygen atom (1.226(3) Å) is appreciably shorter than that with the coordinated atom (1.286(3) Å). The OCO angles for the κ2 and κ1 ligands also differ noticeably (118.9(3)° and 126.6(3)°, respectively), which is due to the stress in the four-membered ring RuOCO. These specific features of the structures of two carboxylate ligands at the Ru atom were observed in the earlier characterized acetate [28], pivalate [9], and benzoate complexes [29].
Complex II was synthesized by the consecutive treatment of nickel acetate with cymantrenecarboxylate acid (2 equiv) and 1,4-di-tert-butyl-1,4-diazabutadiene (L2). The formed green crystals are poorly soluble in benzene, dichloromethane, methanol, and acetone.

According to the XRD data, the Ni–N bonds in the structure of compound II (2.086(3) and 2.080(3) Å) are close to those in the DipyNi(OOCCMe3)2 complex (2.033(3)–2.059(4) Å) [30]. The Ni–O bonds arranged in the trans position to the oxygen atoms are by 0.1 Å shorter than the corresponding bonds in the trans position to the diazabutadiene ligand (2.066(3) and 2.072(3) Å for the shorter bonds and 2.197(3) and 2.153(3) Å for the longer bonds). A similar but less pronounced difference in the Ni–O distances is observed in the benzoate complex DipyNi(OOCPh)2 (Ni–O is 2.145 and 2.155 Å in the trans position to the nitrogen atoms and 2.051 and 2.075 Å in the trans position to the oxygen atoms) [31]. In the complex with more donor pivalates DipyNi(OOCCMe3)2, there are almost no differences in the Ni–O distances and the corresponding bonds are 2.104(4)–2.137(3) Å, whereas monodentate cymantrenecarboxylate in the Ni[(OOCC5H4)-Mn(CO)3]2[O(H)Me]4 complex forms the Ni–O bond equal to 2.0305(14) Å [21]. In the noncoordinated ligand L2 in the trans conformation, the C=N bond is 1.269(2) Å and C–C is 1.477(2) Å [32]. For the coordination of the nickel atom, ligand L2 gains the cis conformation, but this almost does not affect the bond lengths in this ligand (C–C 1.472(6), C=N 1.265(5) and 1.260(5) Å).
Thus, the heterometallic nickel(II) and ruthenium(II) complexes with the sterically loaded ligands (4-isopropyl-1-methylbenzene (L1) and 1,4-di-tert-butyl-1,4-diazabutadiene-1,3 (L2)) containing the IR labels as the cymantrenyl group were synthesized.
REFERENCES
Drabina, P., Funk, P., Ruzicka, A., et al., Transition Met. Chem., 2010, vol. 35, p. 363.
Mesbah, A., Jacques, S., Rocca, E., et al., Eur. J. Inorg. Chem., 2011, p. 1315.
Uddin, N., Sirajuddin, M., Uddin, N., et al., Spectrochim. Acta Mol. Biomol. Spectrosc., 2015, vol. 140, p. 563.
Dobson, A. and Robinson, S.D., Inorg. Chem., 1977, vol. 16, p. 137.
Lynam., J.M., Welby, C.E., and Whitwood, A.C., Organometallics, 2009, vol. 28, p. 1320.
Hiett, N.P., Lynam, J.M., Welby, C.E., et al., J. Organomet. Chem., 2011, vol. 696, p. 378.
Gawin, R., Makal, A., Woźniak, K., et al., Angew. Chem., Int. Ed. Engl., 2007, vol. 46, p. 7206.
Lipovska, P., Rathouska, L., Šimůnek, O., et al., J. Fluorine Chem., 2016, vol. 191, p. 14.
Arockiam, P.B., Fischmeister, C., Bruneau, C., et al., Angew. Chem., Int. Ed. Engl., 2010, vol. 49, p. 6629.
Liu, J.Y., Cao, G.E., Xu, W., et al., Appl. Organomet. Chem., 2010, vol. 24, no. 10, p. 685.
Gong, M., Zhou, W., Tsai, M.-C., et al., Nat. Commun., 2014, p. 4695.
Haider, A.J., Al-Anbari, R., Sami, H.M., et al., J. Mater. Res. Technol., 2019, vol. 8, no. 3, p. 2802.
Halawy, S.A., Mohamed, M.A., and Abdelkader, A., Arab. J. Chem., 2018, vol. 11, no. 6, p. 991.
Brammer, L., Rivas, J.C.M., Atencio, R., et al., Dalton Trans., 2000, p. 3855.
Murugesapandian, B. and Roesky, P.W., Dalton Trans., 2010, vol. 39, p. 9598.
Orlova, T.Yu., Setkina, V.N., and Kursanov, D.N., J. Organomet. Chem., 1984, vol. 267, p. 309.
Wang, Y.-P., Pang, S.-R., Cheng, H.-Y., et al., J. Organomet. Chem., 2008, vol. 693, p. 329.
van Staveren, D.R., Weyhermuller, T., and Metzler-Nolte, N., Organometallics, 2000, vol. 1, p. 3730.
Ageshina, A.A., Uvarova, M.A., and Nefedov, S.E., Russ. J. Inorg. Chem., 2015, vol. 60, p. 1085. https://doi.org/10.1134/s0036023615090028
Pasynskii, A.A., Shapovalov, S.S., Gordienko, A.V., et al., Russ. J. Coord. Chem., 2011, vol. 37, p. 127. https://doi.org/10.1134/s1070328411020072
Van Koten, G. and Vrieze, K., Adv. Inorg. Chem., 1982, vol. 21, p. 151.
Shapovalov, S.S., Pasynskii, A.A., Skabitskii, I.V., et al., Russ. J. Coord. Chem., 2014, vol. 40, p. 77. https://doi.org/10.1134/s1070328414020092
Chang, Y.-Ch., Lee, Y.-Ch., and Chang, M.-F., J. Organomet. Chem., 2016, vol. 808, p. 23.
Sheldrick, G.M., SADABS (2016/2), Göttingen: Univ. of Göttingen, 2016.
Sheldrick, G.M., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, p. 3.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., et al., J. Appl. Crystallogr., 2009, vol. 42, p. 339.
Spek, A.L., Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, vol. 65, p. 148.
Kossoy, E., Diskin-Posner, Y., Leitus, G., and Milstein, D., Adv. Synth. Catal., 2012, vol. 354, p. 497.
Lo, V.K.Y., Guo, Z., Choi, M.K.W., et al, J. Am. Chem. Soc., 2012, vol. 134, p. 7588.
Eremenko, I.L., Nefedov, S.E., Sidorov, A.A., et al., Inorg. Chem., 1999, vol. 38, no. 17, p. 3764.
Baruah, A.M., Karmakar, A., and Baruah, J.B., Polyhedron, 2007, vol. 26, p. 4479.
Haaf, M., Schmiedl, A., Schmedake, T.A., et al., J. Am. Chem. Soc., 1998, vol. 120, p. 12714.
ACKNOWLEDGMENTS
The studies were carried out using the equipment of the Center for Collective Use of Physical Methods of Investigation at the Kurnakov Institute of General and Inorganic Chemistry (Russian Academy of Sciences).
Funding
This work was supported by the Russian Science Foundation, project no. 18-73-10206.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Shapovalov, S.S., Skabitskii, I.V. Ruthenium and Nickel Complexes with Cymanthrenenecarboxylic Acid: Synthesis and Structures. Russ J Coord Chem 47, 330–334 (2021). https://doi.org/10.1134/S1070328421050055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328421050055