Abstract
The heterospin copper(II) complex, ((pyridin-2-ylmethylene)-4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl)-3,6-di-tert-butylcatecholatocopper(II) (I), is synthesized and characterized by IR spectroscopy, magnetochemistry, EPR, and X-ray diffraction analysis. The one-electron oxidation of complex I by AgBF4 affords the biradical copper(I) complex: bis[((pyridin-2-ylmethylene)-4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl)]copper(I) (II). An analysis of the parameters of isotropic EPR spectra of complexes I and II indicates that they are biradicals with the fast (I) and intermediate (II) exchange interaction between the radical centers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The search for new heterospin metal complexes bearing a paramagnetic ligand along with a magnetic metallocenter is one of the actively developed trends in the design of molecular magnets [1, 2]. Nitroxyl (nitronylnitroxyl) radicals [1–4] and o-semiquinone radical anions [5–11] are most frequently used as such radical ligands. Many paramagnetic ligands have been synthesized to the present time: functionalized derivatives of 2,2,6,6-tetramethylpiperidine-1-oxyl containing the nitroxyl magnetic center and the group providing coordination with the metal [3, 4]. One of these ligands, (pyridin-2-ylmethylene)-4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl (L1) bearing the chelate iminopyridyl site capable of binding strongly with the metallocenter, has first been proposed [4] for the EPR study of metal–nitroxyl exchange interactions in the solution. The whole series of the manganese, cobalt, nickel, and copper complexes was studied using this procedure [12–15]. However, none molecular structure of the studied complexes was determined. We have recently synthesized the heterospin bis-o-semiquinone cobalt complex with this radical ligand and characterized them by X-ray diffraction analysis and magnetochemistry [16].
The molecular structure and magnetic properties of the copper(II) catecholate complex with ligand L1, ((pyridin-2-ylmethylene)-4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl)-3,6-di-tert-butylcatecholatocopper(II) (I), were studied in this work along with the investigation by the EPR method.
EXPERIMENTAL
All manipulations with the complexes were carried out in evacuated ampoules using organic solvents purified according to standard procedures [17]. The initial ligands [12, 18] and copper bis-o-semiquinolate [19] were synthesized according to known literature data.
EPR spectra were recorded om a Bruker EMX spectrometer equipped with a temperature attachment, an NMR magnetometer, and a precision frequency meter. The parameters of the EPR spectra were obtained by simulation in the EasySpin program package [20] using line broadening compensation due to the fast-motion regime effect [21].
TheX-ray diffraction analysis of the crystalline sample of complex I (0.44 × 0.11 × 0.10 mm) was carried out on a Bruker D8 Quest diffractometer (ω scan mode, МоKα radiation, λ = 0.71073 Å, T = 100 K, 2θ = 56.93°). Experimental sets of intensities were measured and integrated, an absorption correction was applied, and structure refinement was performed using the APEX3 [22], SADABS [23], and SHELX [24] program packages. The crystals of complex I (C29H41CuN3O3 ∙ 1/2C7H8) are monoclinic, space group P21/n, a = 12.3955(4), b = 12.7099(4), c = 20.2488(6) Å, β = 105.2380(10)°, V = 3077.95(17) Å3, Z = 4, ρcalcd = 1.272 mg/m3, μ = 0.746 mm–1. The number of measured reflections was 36 942, and 7712 independent reflections (Rint = 0.0269) were used to refine 463 structure parameters by full-matrix least squares for \(F_{{hkl}}^{2}\) in the anisotropic approximation for non-hydrogen atoms. Hydrogen atoms were placed in the geometrically calculated positions and refined isotropically with the fixed thermal parameters U(H)iso = 1.2U(C)equiv (U(H)iso = 1.5U(C)equiv for methyl groups).
An analysis of the Fourier electron density synthesis showed that the iminopyridinate ligand and solvate toluene molecule in complex I were disordered over two positions each. The SADI, DFIX, ISOR, EADP, and RIGU instructions were used to restrict the geometric characteristics and anisotropic parameters of atomic shifts when refining the disordered fragments. After the final refinement, wR2 = 0.0827 and S(F 2) = 1.027 for all reflections (R1 = 0.0316 for I > 2σ(I)). The residual electron density maximum and minimum were 0.50 and –0.53 e/Å3.
The structure of complex I was deposited with the Cambridge Crystallographic Data Centre (CIF file CCDC no. 1882883; ccdc.cam.ac.uk/structures).
The magnetic susceptibility (χ) of a polycrystalline sample was measured on an MPMSXL SQUID magnetometer (Quantum Design) in the temperature range from 2 to 300 K at a magnetic field intensity of 5 kOe. The paramagnetic components of the magnetic susceptibility were determined taking into account the diamagnetic contribution estimated from Pascal’s constants. The effective magnetic moment (μeff) was calculated by the equation [3kχT/\(\left( {{{N}_{{\text{A}}}}\mu _{{\text{B}}}^{{\text{2}}}} \right)\)]1/2, where NA, μB, and k are Avogadro’s number, the Bohr magneton, and the Boltzmann constant, respectively.
Synthesis of complex I. A solution of L1 (0.0260 g, 10–4 mol) in toluene (15 mL) was added to a solution of bis(3,6-di-tert-butylbenzosemiquinolate-1,2)copper(II) (0.0504 g, 10–4 mol) in toluene (30 mL) placed in an evacuated tube. The reaction mixture was kept at room temperature for 24 h. The dark blue crystalline product that formed was filtered off, washed with cold toluene, and dried in vacuo. The product contained one toluene molecule per two complex molecules. The yield was 0.055 g (93%).
IR (ν, cm–1): 509 w, 533 w, 575 m, 593 m, 651 s, 693 s, 715 w, 742 s, 762 s, 777 s, 808 m, 887 w, 923 m, 938 s, 977 s, 1026 s, 1043 w, 1067 w, 1082 w, 1107 m, 1145 m, 1160 w, 1177 m, 1194 w, 1207 w, 1258 s, 1279 m, 1292 w, 1305 w, 1314 w, 1347 w, 1353 w, 1362 w, 1378 s, 1402 s, 1447 w, 1462 s, 1482 w, 1493 w, 1553 w, 1568 w, 1596 s, 1633 m, 2854 s, 2924 s, 2950 s.
For C32.5H46N3O3Cu | ||||
Anal. calcd., % | C, 66.13 | H, 7.86 | N, 7.12 | Cu, 10.77 |
Found, % | C, 67.15 | H, 7.98 | N, 7.05 | Cu, 10.63 |
Complex I', ((pyridin-2-ylmethylene)-4-aminocyclohexyl)-3,6-di-tert-butylcatecholatocopper(II), was synthesized according to a procedure similar to that for complex I but using the ligand with the cyclohexyl substituent (L1') (0.019 g, 10–4 mol) instead of L1. Similar to complex I, the product contained one toluene molecule per two complex molecules. The yield was 0.046 g (89%).
IR (ν, cm–1): 550 w, 621 w, 697 w, 722 w, 760 m, 846 w, 887 w, 922 w, 964 w, 994 w, 1042 s, 1078 w, 1135 w, 1247 m, 1342 s, 1376 s, 1459 s, 1465 m, 1561 s, 1640 s, 2852 s, 2923 s, 2944 s.
For C29.5H40N2O2Cu | ||||
Anal. calcd., % | C, 68.38 | H, 7.78 | N, 5.41 | Cu, 12.26 |
Found, % | C, 68.75 | H, 8.09 | N, 5.35 | Cu, 12.18 |
Synthesis of complex II (bis[((pyridin-2-ylmethylene)-4-amino-2,2,6,6-tetramethylpiperidine-1-oxyl)]-copper(I). (a) THF (5 mL) was condensed in vacuo to a mixture of complex I (0.0295 g, 5 × 10–5 mol) and AgBF4 (0.0097 g, 5 × 10–5 mol). The reaction mixture was examined by the EPR method without isolation of the complex.
(b) THF (15 mL) was condensed in vacuo to a mixture of CuCl (0.0050 g, 5 × 10–5 mol), KPF6 (0.0092 g, 5 × 10–5 mol), and L1 (0.0260 g, 10–4 mol). The gradual transformation of the signal of L1 into the signal of complex II was observed with time in the EPR spectrum. The product was not isolated in the individual form.
RESULTS AND DISCUSSION
The catecholate complexes containing the TEMPO–iminopyridine ligand L1 (I) and its non-radical analog (cyclohexyliminopyridine ligand L1') (I') were synthesized by the substitution reaction from copper(II) bis-o-semiquinolate [19].

Complex I isolated in the individual form represents a dark blue crystalline substance highly soluble in THF and dichloromethane and completely insoluble in toluene and saturated hydrocarbons. Its composition and structure were characterized by the X-ray diffraction analysis, EPR spectroscopy, IR spectroscopy, and magnetochemistry methods.
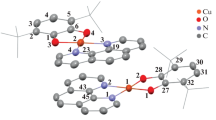
According to the X-ray diffraction analysis data, compound I is the copper(II) complex in which the Cu2+ cation is bound to two oxygen atoms of the catecholate ligand and two nitrogen atoms of the iminopyridinate ligand (Fig. 1). The symmetrically independent part of the crystal cell contains the complex molecule and also the centrosymmetric solvate toluene molecule. Thus, compound I crystallizes in the form of (Cat)Cu(TEMPO-ImPy) ∙ 1/2(C7H8). The coordination polyhedron of the Cu(II) atom is a distorted square. The dihedral angle between the O(1)C(1)C(2)O(2) and N(1)C(19)C(2)N(2) planes of the quinone and iminopyridinate fragments is 13.4(2)°. The Cu(II) atom does not almost deviate (deviates by 0.02(2) Å only) from the O(1)O(2)N(1)N(2) mean plane, and the sum of bond angles at the copper atom is 361.1(5)°, which is close to the value for the ideal planar square structure (360°). The C–O and С–С bond lengths in the quinone fragments (1.344(2)–1.345(2) and 1.421(2) Å, respectively) make it possible to unambiguously characterize the structure of the quinone ligand as a catecholate one. The Cu–O bond lengths (1.869(2)–1.880(2) Å) also correspond to the values characteristic of the copper(II) catecholate complexes [25].
The iminopyridinate ligand in complex I is completely disordered over two positions in a ratio of ~50 : 50%. Since the geometric characteristics of the disordered fragments are similar, the bond lengths and angles are presented for one position of the ligand. The Cu–N(1) and Cu–N(2) bond lengths are comparable being 2.04(2) and 2.055(9) Å, respectively. The N(2)–C(20) bond (1.274(9) Å) is much shorter than C(19)–C(20) (1.453(5) Å), which is characteristic of ligands of this type. The TEMPO fragment in complex I exists in the chair conformation: the dihedral angle between the C(21)C(22)C(25) and C(22)C(23)C(24)C(25) planes is 55.4(3)°, and that between C(22)C(23)C(24)C(25) and C(23)N(3)C(24) is 32.8(5)°. The heterocycle is nearly perpendicular to the iminopyridinate fragment: the dihedral angle between the N(1)C(19)C(20)N(2) and C(21)C(22)C(23)N(3)C(24)C(25) planes is 82.5(3)°.
An analysis of the crystal packing in complex I showed intermolecular O···H interactions between the oxygen atom of the nitroxyl group and the hydrogen atom of the CH groups of the iminopyridine fragments of the adjacent molecules. The O···HIm distance (1.93(4) Å) is significantly shorter than the sum of the van der Waals radii of O and H (2.7 Å). The O···HРy distance is longer than O···HIm and is equal to 2.50(4) Å. Numerous intermolecular H···H interactions are also observed in complex I, and the H···H distances lie in a wide range of 2.16(5)–2.39(5) Å.
The IR spectrum of complex I exhibited intense ν(C–O) bands at 1246 and 1258 cm–1 corresponding to two ordinary CO bonds of the catecholate fragment [25]. The ν(C=N) band at 1635 cm–1 is also observed and can be assigned to the CN double bond of the coordinated iminonitroxyl fragment [14].
Complex I is paramagnetic. The value of µeff is 2.47 µB at 300 K, remains almost unchanged with decreasing temperature to 50 K, and then decreases to 1.77 µB at 2 K (Fig. 2). The high-temperature value of µeff is consistent with the theoretical spin-only value (2.45 µB) for two paramagnetic centers with the spins S = 2: one Cu2+ ion and one nitroxyl. A decrease in µeff at temperatures below 50 K indicates antiferromagnetic exchange interactions between the magnetic centers, and the value of µeff at 2 K agrees with the theoretical value for one paramagnetic center. According to the structural data, these channels of the intermolecular antiferromagnetic exchange in complex I can be O···H intermolecular interactions between the oxygen atom of the nitroxyl group and the hydrogen atom of the CH groups of the iminopyridine fragments. Thus, paramagnetism of the complex at low temperatures is caused by the spins of the Cu2+ ions only. An analysis of the µeff(T) dependence was carried out in terms of the model for the exchange-bonded dimer (H = –2JS1S2 and gR = 2 were fixed taking into account the contribution of the Cu2+ ions to the magnetic susceptibility according to the Curie law). The optimum values of the intermolecular exchange interaction parameter (J') and gCu are 4.1 cm–1 and 2.04, respectively.
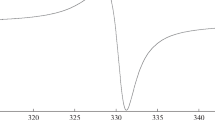
The EPR spectrum of complex I (Fig. 3a) is a quartet (1 : 1 : 1 : 1) caused by the hyperfine coupling (HFC) with the 63Cu and 65Cu magnetic isotopes of the copper(II) atom (gi = 2.0552, aCuFootnote 1 = 4.8 mT, where aCu is the HFC constant). The spectrum also contains the impurity 1 : 1 : 1 triplet signal from the initial iminopyridine ligand (gi = 2.0057, aN = 1.55 mT), whose integral intensity is lower than 1%.
The observed values gi = 2.0552 and aCu = 4.8 mT for complex I differ substantially from the corresponding values characteristic of similar catecholate copper(II) complexes with the diaza ligands [25]. The isotropic EPR spectrum of the similar catecholate complex with diamagnetic iminopyridine ligand L1' (I') (Fig. 3b) has the EPR spectral parameters (gi = 2.0927, aCu = 9.5 mT) characteristic of the copper(II) derivatives. The hyperfine splitting on the 14N nitrogen nuclei of the iminopyridine fragment is additionally observed (aN(1) = 1.14 mT, aN(2) = 1.02 mT).
The EPR spectral parameters of complex I are characteristic of the biradical compounds, whose electron–electron coupling constant (J) considerably exceeds the HFC constant (J\( \gg \)a) [26]. The fast exchange between the radical centers in these systems leads to averaging their parameters. The observed experimental values for complex I satisfy this rule: the value gi = 2.0552 is ~1/2 of the sum of gi = 2.0927 and 2.0057. The HFC constant equal to 4.8 mT with the magnetic isotopes is halved over the corresponding aCu = 9.5 mT in complex I'. The absence of a pronounced hyperfine structure on the 14N nitrogen nuclei of the nitroxyl group (the expected value is aN ≈ 0.77 mT) and iminopyridine fragment (the expected value is aN ≈ 0.55 mT) in the EPR spectrum of complex I is related, most likely, to the fact that the value of hyperfine splitting is substantially lower than the linewidth (~2.0 mT).
Thus, complex I is biradical with the localization of the paramagnetic centers on the copper(II) ion and nitroxyl group of the TEMPO fragment.
Since the dioxolene complexes are characterized by redox activity [27], we made an attempt of the one-electron oxidation of the catecholate ligand in complex I to obtain the o-semiquinone derivative containing already three radical centers (о-semiquinone, nitroxyl, and Cu(II)).

However, the oxidation of complex I does not give the desired result. The treatment of a solution of complex I in THF with AgBF4 leads to the appearance of the EPR spectrum (Fig. 3c) typical of bis(nitroxyl) biradicals with J ≈ a [26]. No hyperfine interaction with the 63Cu and 65Cu magnetic isotopes is observed. The EPR spectrum observed corresponds to the Cu(I) bis(iminopyridine) complex II+B\({\text{F}}_{4}^{ - }\) containing two radical ligands L1 coordinated with the Cu+ ion. Its formation is possible due to the ligand exchange between the primary oxidation product and initial complex I.

Similar bis(ligand) complex II+P\({\text{F}}_{6}^{ - }\) containing the identical cation is formed directly by the reaction of L1 with CuPF6. The parameters of its isotropic EPR spectrum coincide completely with the corresponding values for complex II+BF4.

The temperature dependences of the EPR spectra of complexes II+B\({\text{F}}_{4}^{ - }\) and II+P\({\text{F}}_{6}^{ - }\) completely correspond to the behavior of the nitroxyl biradical systems characterized by the temperature dependence of the J parameter [26]. An analysis of the temperature dependence of the parameters of the EPR spectra of II+ shows that the J constant decreases monotonically from J ≈ 26 Oe at 300 K to J ≈ 7 Oe at 220 K and lower.
Thus, two new heterospin complexes were synthesized on the basis of radical TEMPO–iminopyridine ligand L1: ((pyridin-2-ylmethylene)-4-amino-2,2,-6,6-tetramethylpiperidine-1-oxyl)-3,6-di-tert-butylcatecholatocopper(II) (I) and bis[((pyridin-2-ylmethylene)-4-amino-2,2,6,6-tetramethylpiperidi-ne-1-oxyl)]copper(I) (II+B\({\text{F}}_{4}^{ - }\)). An analysis of the parameters of the isotropic EPR spectra of complexes I and II shows that they are biradicals with the fast (J \( \gg \)a, I) or intermediate (J ≈ a, II) exchange interaction between the radical centers.
Notes
Here in after, aCu implies \({{a}_{{^{{63}}{\text{Cu}}}}}\) and \({{a}_{{^{{65}}{\text{Cu}}}}}\) = 1.07.
REFERENCES
Ovcharenko, V., Metal-Nitroxide Complexes: Synthesis and Magnetostructural Correlations, Ch. 13, Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds, Wiley, 2010, p. 461.
Fedin, M.V., Veber, S.L., Bagryanskaya, E.G., et al., Coord. Chem. Rev., 2015, vols. 289−290, p. 341.
Eaton, S.S., Coord. Chem. Rev., 1978, vol. 26, p. 20.
Eaton, S.S. and Eaton, G.R., Coord. Chem. Rev., 1988, vol. 83, p. 29.
Pierpont, C.G. and Buchanan, R.M., Coord. Chem. Rev., 1981, vol. 38, p. 45.
Pierpont, C.G., Coord. Chem. Rev., 2001, vols. 219−221, p. 415.
Pierpont, C.G., Coord. Chem. Rev., 2001, vols. 216–217, p. 99.
Hendrickson, D.N. and Pierpont, C.G., Valence Tautomeric Transition Metal Complexes. Spin Crossover in Transition Metal Compounds II, Berlin: Springer, 2004, vol. 234, p. 63.
Pierpont, C.G. and Lange, C.W., The Chemistry of Transition Metal Complexes Containing Catechol and Semiquinone Ligands. Progress in Inorganic Chemistry, New York: Wiley, 2007, р. 331.
Pierpont, C.G. and Kelly, J.K., Coordination Chemistry of o-Semiquinones. PATAI’S Chemistry of Functional Groups, New York: Wiley, 2009.
Poddel’sky, A.I., Cherkasov, V.K., and Abakumov, G.A., Coord. Chem. Rev., 2009, vol. 253, p. 291.
Boymel, P.M., Eaton, G.R., and Eaton, S.S., Inorg. Chem., 1980, vol. 19, no. 3, p. 727.
Boymel, P.M., Braden, G.A., Eaton, G.R., et al., Inorg. Chem., 1980, vol. 19, no. 3, p. 735.
Hard, S. and Eaton, G.R., J. Magn. Res., 1983, vol. 51, p. 470.
More, J.K., More, K.M., Eaton, G.R., et al., J. Am. Chem. Soc., 1984, vol. 106, p. 5395.
Zolotukhin, A.A., Bubnov, M.P., Arapova, A.V., et al., Inorg. Chem., 2017, vol. 56, p. 14751.
Armarego, W.L.F. and Chai, C., Purification of Laboratory Chemicals, Butterworth-Heinemann, 2013.
Kim, S., Kim, E., Lee, H.J., et al., Polyhedron, 2014, vol. 69, p. 149.
Abakumov, G.A., Lobanov, A.V., Cherkasov, V.K., et al., Inorg. Chim. Acta, 1981, vol. 49, p. 135.
Stoll, S. and Schweiger, A., J. Magn. Res., 2006, vol. 178, p. 42.
Kozhanov, K.A., Bubnov, M.P., Teplova, I.A., et al., J. Mol. Struct., 2017, vol. 1147, p. 541.
APEX3. Version 2016-9, Madison: Bruker AXS Inc., 2016.
Krause, L., Herbst-Irmer, R., Sheldrick, G.M., et al., J. Appl. Crystallogr., 2015, vol. 48, p. 3.
Sheldrick, G.M., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, p. 3.
Davidson, R.A., Hao, J., Rheingold, A.L., et al., Polyhedron, 2017, vol. 133, p. 348.
Buchachenko, A.L. and Vasserman, A.M. Stabil’nye radikaly. Elektronnoe stroenie, reaktsionnaya sposobnost’ i primenenie (Stable Radicals. Electronic Structure, Reactivity, and Applications), Moscow: Khimiya, 1973.
Abakumov, G.A., Cherkasov, V.K., Nevodchikov, V.I., et al., Izv. Akad. Nauk., Ser. Khim., 1996, no. 2, p. 464.
ACKNOWLEDGMENTS
The X-ray diffraction studies were carried out in the framework of the state task (theme no. 44.2, registration no. AAAA-A16-116122110053-1) using the scientific equipment of the Center for Collective Use “Analytical Center of the Razuvaev Institute of Organometallic Chemistry, Russian Academy of Sciences.”
Funding
This work was supported by the Russian Science Foundation, grant no. 14-13-01296.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Cherkasova, A.V., Kozhanov, K.A., Zolotukhin, A.A. et al. Heterospin Copper(II) Catecholate Complex with the TEMPO–Iminopyridine Ligand. Russ J Coord Chem 45, 489–495 (2019). https://doi.org/10.1134/S1070328419070029
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328419070029