Abstract
1,2-Benzenedicarboxylic (phthalic) acid (1,2-BdcН2) and bipyridine ligands are used for the synthesis of the Zn(II) and Cd(II) coordination polymers. As a result, the target assembling of four complexes is carried out. The structures of the synthesized complexes are studied by X-ray diffraction analysis. Three of the obtained compounds are new coordination polymers [Zn2(1,2-Bdc)2(Bpe)2]n · 0.25n(Dmf) · 0.25nH2O (I), [Zn2(1,2-Bdc)2(Bpp)2]n (II), and [Cd(1,2-Bdc)(Bpp)(H2O)]n (III) (Bpe is bis(4-pyridyl)ethane, Bpp is 1,2-bis(4-pyridyl)propane, and Dmf is dimethylformamide) (CIF files CCDC nos. 1824264 (I), 1824265 (II), and 1824266 (III)), and the fourth one is the known Cd(II) compound [Cd(1,2-Bdc)(H2O)]n. The study of the structures of compounds I–III reveals the dimensionalities of the polymers: 3D for the Zn(II) polymer and 2D for the Cd(II) polymer. Different shapes of the coordination polyhedra of Zn(II) are found in compounds I and II, and different coordination modes of the doubly deprotonated ligand (1,2-Bdc)2– to the metals are observed in compounds I–III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the recent decades the design and efficient synthesis of coordination polymers based on the self-assembling of metal ions and mixed organic ligands remain to be among the most demanded problems, including studies of the materials with noncentrosymmetric structures [1, 2]. A reasonable choice of organic ligands is one of the foundations in the strategy of designing coordination polymers with intriguing topologies and promising properties. A combination of various carboxylic acids with aromatic N‑donor ligands of the bipyridine type, which can function as bridging ligands capable of forming high-dimensional architectures, is widely used in order to change the dimensionality of coordination compounds [3–5]. Among the bipyridine type ligands, flexible ligands such as 1,2-bis(4-pyridyl)ethane (Bpe) and 1,2-bis(4-pyridyl)propane (Bpp) are of special interest. They can take various conformations due to relative orientations of the methylene groups between two pyridine rings [6]. An analysis of the Cambridge Structural database (CSD) [7] shows that 27 compounds of transition metals with the 1,2-dicarboxylic acid residues (1,2-Bdc)2– and bipyridine type ligands (bipyridine (Bipy), Bpe, Bpp, and bis(4-pyridyl)ethene) have been registered to the present time, and 21 of them are compounds with Biру. These are eight Zn(II) complexes [8–13] and two Cd(II) compounds [14, 15], and only five compounds contain no Bipy [8–10]. Some compounds of this class crystallize in noncentrosymmetric space groups assuming the formation of materials with useful physical properties such as the second harmonic generation and segneto-, piezo-, and pyroelectricity. As a result, these complexes can be used for electronic and nonlinear optical devices, signal processing, and information storage [16, 17].
For the purpose of an efficient synthesis of functional coordination polymers, we focused attention on the Zn(II) and Cd(II) compounds with 1,2-benzenedicarboxylic acid containing Bpe and Bpp. As a result, we synthesized four compounds, three of which are new coordination polymers [Zn2(1,2-Bdc)2(Bpe)2]n · 0.25n(Dmf) · 0.25nH2O (I), [Zn2(1,2-Bdc)2(Bpp)2]n (II), and [Cd(1,2-Bdc)(Bpp)(H2O)]n (III) (Dmf is dimethylformamide), and the fourth one is the known Cd(II) compound [Cd(1,2-Bdc)(H2O)]n [18]. We failed to obtain a Cd(II) compound with (1,2-Bdc)2– and Bpe but succeeded in preparing the new Zn(II) compound with (1,2-Bdc)2– and Bpp, although the CSD already contains two compounds of this metal with the same ligands [8, 9]. In this work we continue to study transition metal compounds with carboxylic acids and ligands of the bipyridine class [19].
EXPERIMENTAL
Compounds I–III were synthesized under hydrothermal conditions.
Synthesis of compound I. A mixture of 1,2-BdcH2 (0.18 g, 1 mmol), Zn(BF4)2 · nH2O (0.24 g, 1 mmol), Bpe (0.18 g, 1 mmol), water (3 mL), methanol (3 mL), and Dmf (5 droplets) was sealed in a 8-mL reactor with the Teflon lining and heated in an autoclave at 120°C for 48 h. Then the autoclave was slowly cooled to room temperature. Colorless crystals suitable for X-ray diffraction analysis were filtered off from the solutions. The crystals were soluble in methanol, ethanol, and Dmf and insoluble in ether. The yield was 21%.
For C40.75H34.25N4.25O8.50Zn2 | |||
Anal. calcd., % | C, 83.57 | H, 5.94 | N, 8.40 |
Found, % | С, 84.17 | Н, 6.00 | N, 8.81 |
Synthesis of compoundII. A mixture of 1,2-BdcH2 (0.18 g, 1 mmol), Zn(BF4)2 · nH2O (0.24 g, 1 mmol), Bpp (0.15 g, 1 mmol), water (3 mL), methanol (3 mL), and Dmf (5 droplets) was sealed in a 8-mL reactor with the Teflon lining and heated in an autoclave at 120°C for 48 h. Then the autoclave was slowly cooled to room temperature. Colorless crystals suitable for X-ray diffraction analysis were filtered off from the solutions. The crystals were soluble in methanol, ethanol, and Dmf and insoluble in ether. The yield was 25%.
For C42H36N4O8Zn2 | |||
Anal. calcd., % | C, 68.73 | H, 4.94 | N, 6.40 |
Found, % | C, 69.59 | H, 5.00 | N, 6.63 |
Synthesis of compound III. A mixture of 1,2-BdcH2 (0.18 g, 1 mmol), Cd(BF4)2 · nH2O (0.24 g, 1 mmol), Bpp (0.15 g, 1 mmol), water (3 mL), methanol (3 mL), and Dmf (10 droplets) was sealed in a 8-mL reactor with the Teflon lining and heated in an autoclave at 120°C for 24 h. Then the autoclave was slowly cooled to room temperature. Colorless crystals suitable for X-ray diffraction analysis were filtered off from the solutions. The crystals were soluble in methanol, ethanol, and Dmf and insoluble in ether. The yield was 30%.
For C21H20N2O5Cd | |||
Anal. calcd., % | C, 65.73 | H, 4.83 | N, 6.92 |
Found, % | C, 66.12 | H, 5.09 | N, 7.16 |
All chemical substances and solvents were purchased from Aldrich and used as received.
X-ray diffraction analysis of compoundsI–III. The X-ray diffraction data were obtained on an Xcalibur E diffractometer (graphite monochromator, MoKα radiation) at room temperature. The unit cell parameters were determined and the experimental data were processed using the CrysAlis Oxford Diffraction Ltd. program [20]. The structures of compounds I–III were determined by direct methods and refined by least squares in the anisotropic full-matrix approximation for non-hydrogen atoms (SHELX-97) [21]. The experimental data obtained from a twin crystal were used for the structure determination of compound II. The disordering over two positions in a ratio of 1 : 1 was determined in the structure of compound III for the propane fragment of the Bpp ligand. The positions of the hydrogen atoms related to the oxygen atoms were obtained from the difference Fourier syntheses, and the positions of all other hydrogen atoms were calculated geometrically. The experimental and structure refinement characteristics for compounds I–III are presented in Table 1. The interatomic distances and bond angles in the coordination nodes are given in Table 2. The hydrogen bonding geometry is presented in Table 3. The positions and thermal parameters of atoms of compounds I–III were deposited with the CSD in the Cambridge Crystallographic Data Centre (CIF files CCDC nos. 1824264 (I), 1824265 (II), 1824266 (III); deposit@ccdc.ca.ac.uk or www.ccdc.cam.ac.uk/conts/retrieving.html).
RESULTS AND DISCUSSION
The X-ray diffraction data showed that the reactions of the Zn(II) and Cd(II) salts containing \(\text{BF}_{4}^{-} \) anions with 1,2-BdcH2 and Bpe or Bpp afforded four coordination polymers. Compounds I–III (see Scheme 1) are new and contain both organic ligands, whereas the known coordination polymer was formed in the reaction of the Cd(II) salt, 1,2-BdcH2, and Bpe [18]. All materials obtained are coordination polymers with different compositions, topologies, and dimensionalities.

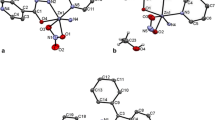
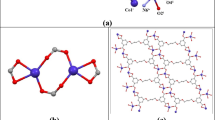
The independent part of the unit cell of compound I contains a fragment of the coordination 3D polymer [Zn2(1,2-Bdc)2(Bpe)2]n formed by two crystallographically independent atoms Zn(1) and Zn(2), two doubly deprotonated ligands (1,2-Bdc)2–, three neutral ligands Bpe (two of which are centrosymmetric), and crystallization molecules Dmf and H2O in the metal to Dmf to H2O ratio equal to 2 : 0.25 : 0.25. The coordination polyhedra of both atoms Zn(1) and Zn(2) in compound I are tetrahedra formed by the donor set of atoms N2O2, whose nitrogen atoms belong to two crystallographically different Вpe ligands and the oxygen atoms belong to two different (1,2-Bdc)2– ligands (Fig. 1). In the coordination polyhedra of the Zn(1) and Zn(2) atoms, the interatomic distances Zn–O (1.945(4)–1.969(5) Å) and Zn–N (2.017(6)–2.036(6) Å) (Table 2) correspond to the values found for the compounds with the tetrahedral environment of the zinc atoms (coordination number 4) [8]. An analysis of the CSD shows that the coordination number of Zn(II) atoms in the Bipy-containing compounds of this class can take different values (both 5 and 6) [11, 12]. In the coordination polymer of compound I, two independent ligands (1,2-Bdc)2– coordinate to the metal via the bidentate-bridging mode (Fig. 1), although it is known that this ligand can coordinate via diverse modes: monodentate, bidentate-chelating, and various bridging modes (μ2, μ3, μ4) [22, 23]. As a result, coil chains arranged along the a parameter of the unit cell are formed in the crystal of compound I, and the interatomic Zn(1)···Zn(2)* distances between the atoms linked by the (1,2-Bdc)2– ligands are 6.367 and 6.575 Å (Fig. 2a). In compound I, the Bpe ligands coordinate to the metal atoms via the bidentate-bridging mode through the terminal nitrogen atoms (Fig. 2b) linking both Zn(1) with Zn(2) (Fig. 2b), Zn(1) with Zn(1)*, and Zn(2) with Zn(2)*. The interatomic Zn(1)···Zn(2), Zn(1)···Zn(1)*, and Zn(2)···Zn(2)* distances are 13.228, 13.279, and 13.383 Å, respectively. Although the Bpe ligands in the crystal structure of compound I differ in symmetry, they are nearly similar. In the noncentrosymmetric ligand, the ССН2СН2С torsion angle containing the ethane fragment is 176° and the dihedral angle between the planes of the aromatic rings is 2.8°. These data do not differ strongly from similar values in the centrosymmetric Вpe ligands. A 2D network involving both the (1,2-Вdc)2– ligands and the noncentrosymmetric Вpe ligand (Fig. 3a) can be distinguished in the crystal of compound I. The network is completed to a 3D framework by the centrosymmetric Вpe ligands (Fig. 3b). The same structure was observed in the coordination 3D polymer [Zn2(1,2-Вdc)2(Вpp)2]n (IV) [8] in which both ligands (1,2-Вdc)2– and Вpp are involved in coordination similarly to compound I to form a tetrahedral environment of two crystallographically independent metal atoms. However, these results differ strongly from those found for the coordination 1D polymer with the same ligands [Zn2(1,2-Вdc)2(Вpp)]n [9] in which Вpp joins two Zn2+ ions into dimers by the dicarboxylate ligands bound into chains. In the 1D polymer [9], the type of the coordination polyhedron of the metal atom differs from those observed in compounds I and IV and the (1,2-Вdc)2– ligands coordinate to the Zn(II) atoms via different modes.
The coordination polymer in the crystal structure of compound I is stabilized by weak hydrogen bonds of the С–Н···О type. The residues of Dmf and H2O crystallization molecules linked by hydrogen bonds are incorporated into free cavities of the coordination 3D polymer (Table 3, Fig. 4a). After the removal of crystallization molecules, the volume of the free cavities in compound I is 1265.1 Å3 (of 27.7% оf the unit cell volume) (Fig. 4b).
The independent part of the unit cell of compound II contains the fragment of the coordination 3D polymer [Zn2(1,2-Вdc)2(Вpр)2]n including two crystallographically independent Zn(1) and Zn(2) atoms, two doubly deprotonated (1,2-Вdc)2– ligands, and two neutral Вpp ligands. The coordination polyhedra of the Zn(1) and Zn(2) metal atoms in compound II differ: a tetrahedron formed by the donor set of atoms N2O2 (nitrogen atoms of two crystallographically different Вpр ligands and oxygen atoms of two different (1,2-Вdc)2–) is observed for Zn(1), and a strongly distorted tetragonal pyramid with the donor set of atoms N2O3 is observed for Zn(2), since the τ index of the tetragonal pyramid polyhedron calculated by the equation τ = β – α/60 [24, 25] is equal to 0.135; i.e., its value is closer to 0 than to 1. In the coordination polyhedron of Zn(2), two nitrogen atoms belong to two crystallographically different Вpр ligands and three oxygen atoms belong to two different (1,2-Вdc)2– (Fig. 5). In the coordination polyhedron of the Zn(1) atom, the interatomic distances are 1.94(1) and 1.89(2) Å for Zn–O and 2.07(1) and 2.03(1) Å for Zn–N. For the Zn(2) atoms the distances are 1.95(1), 2.23(3), and 2.46(3) Å for Zn–O and 2.055(8) and 2.03(5) Å for Zn–N (Table 2). The type of the coordination polyhedron of the Zn(1) atom is similar to the tetrahedra in compounds I and IV, whereas the tetragonal pyramid type observed for Zn(2) is similar to that for the metal atom in the Zn(II) complex with (1,2-ВdcH)– and Вipy [11].
In the coordination Zn(II) polymer (II), two independent (1,2-Вdc)2– ligands coordinate to the metal atoms via different modes. One ligand is bound to two metal atoms via the bidentate-bridging mode involving two oxygen atoms of different carboxyl groups, and the second ligand uses the tridentate chelate-bridging mode and binds the Zn(1) and Zn(2) atoms through one/two oxygen atoms of different carboxyl groups (Fig. 6). The chains along the b parameter of the unit cell are formed in the crystal, and the metal atoms and crystallographically independent (1,2-Вdc)2– ligands alternate in the chains. The Zn(1)···Zn(2) and Zn(1)···Zn(2)* interatomic distances are 6.047 and 6.397 Å, respectively. In the crystal of compound II, the Вpр ligands coordinate to the metal atom via the bidentate-bridging mode (Fig. 5): one of the ligands binds two Zn(1) atoms, and the second ligand binds two Zn(2) atoms. The Zn(1)···Zn(1)* and Zn(2)···Zn(2)* interatomic distances are 13.287 and 12.199 Å, respectively. Both Вpр ligands in the crystal exist in the general position. The С–СН2–СН2–С torsion angles containing the propane fragment are equal to 166° and 171° in one ligand and to 166° and 176° in another ligand. The dihedral angles between the planes of the aromatic rings in these ligands are 109.2° and 98.4°, unlike similar angles in compound I. In the crystal of compound II, the chains the formation of which involves both (1,2-Вdc)2– ligands are completed by the Вpр ligand to the coordination 3D polymer and are stabilized by weak hydrogen bonds С–Н···О (Table 3, Fig. 7a). The volume of the free cavities in compound II (763.5 Å3) is 12.0% of the unit cell volume (Fig. 7b).
The independent part of the unit cell of compound III contains the fragment of the coordination 2D polymer [Сd(1,2-Вdc)(Вpр)]n formed by one crystallographically independent Cd(1) atom, doubly deprotonated (1,2-Вdc)2– ligand, and neutral Вpp ligand. The coordination polyhedron of the Cd(1) atom in compound III is a pentagonal bipyramid formed by the donor set of atoms N2O5. The nitrogen atoms belong to two Вpр ligands, and the oxygen atoms belong to two (1,2-Вdc)2– ligands and coordinated water molecule (Fig. 8). The Cd–O(Вdc), Cd–N(Вpp), and Cd–O(w) interatomic distances are 2.349(3)–2.483(3), 2.349(3) and 2.369(3), and 2.463(3) Å, respectively (Table 2). The coordination polyhedron of the Cd(1) atom in compound III is similar to that found in the Cd(II) complex without Вpp [18] but differs from polyhedra observed in the complexes of this metal containing (1,2-Вdc)2– or (1,2-HВdc)– and Вipy [14, 15].
In the coordination 2D polymer (III), the (1,2-Вdc)2– ligand coordinates to two metal atoms via the tetradentate chelate-bridging mode, i.e., involving both oxygen atoms of the carboxyl groups (Figs. 8, 9). The chains are formed in the crystal along the b parameter of the unit cell, the metal atoms in them are linked by the (1,2-Вdc)2– ligands, and the Cd(1)···Cd(1)* interatomic distance is 6.577 Å. The Вpр ligands in the crystal coordinate to the metal atoms in the same way as in compounds I and II. The Cd(1)···Cd(1)* distance is 14.627 Å (Fig. 8), and the dihedral angle between the planes of the aromatic rings in Bpp (91.5°) differs slightly from the similar angle in compound II.
In the crystal of compound III, the chains the formation of which involves the (1,2-Вdc)2– ligands are completed by the Вpр ligands to the coordination 2D polymer additionally stabilized by weak hydrogen bonds О(w)–Н···О and С–Н···О involving the atoms of the aromatic fragments of the Bpp ligands as proton donors and the oxygen atoms of the carboxyl groups of the (1,2-Вdc)2– ligands as acceptors (Table 3, Fig. 10a). The 2D layers parallel to the ab plane are linked between each other by both O(w)–Н···О hydrogen bonds and weak С–Н···О hydrogen bonds (Table 3, Fig. 10b). The unit cell of compound III contains no free volume.
The reactions of the Zn(II) and Cd(II) salts with 1,2-benzenedicarboxylic acid and Вpe and Вpp ligands of the bipyridine type afforded four compounds, three of which are the new coordination polymers [Zn2(1,2-Вdc)2(Вpe)2]n ∙ 0.25n(Dmf) ∙ 0.25nH2O (I), [Zn2(1,2-Bdc)2(Bpp)2]n (II), and [Cd(1,2-Вdc)(Вpp)(H2O)]n (III). The fourth synthesized Cd(II) compound containing none of organic ligands has been known earlier [18]. The new Zn(II) compound with (1,2-Вdc)2– and Вpp was obtained by the variation of the reaction conditions and solvents, although the compounds of this metal with the same ligands are also known [8, 9].
REFERENCES
Gu, Z.-G., Zhou, X.-H., Jin, Y.-B., et al., Inorg. Chem., 2007, vol. 46, p. 5462.
Xie, Y.-M., Liu, J.-H., Wu, X.-Y., et al., Cryst. Growth Des., 2008, vol. 8, p. 3914.
Biradha, K., Sarkar, M., and Rajput, L., Chem. Commun., 2006, p. 4169.
Manna, S.C., Konar, S., Zangrando, E., et al., Eur. J. Inorg. Chem., 2005, p. 4646.
Li, Z.-G., Wang, G.-H., Jia, H.-Q., et al., CrystEngComm, 2008, vol. 10, p. 983.
Carlucci, L., Ciani, G., Proserpio, D.M., and Rizzato, S., CrystEngComm, 2002, vol. 4, p. 121.
Allen, F.H., Acta Crystallogr., Sect. B: Struct. Sci., 2002, vol. 58, nos. 3−1, p. 380.
Zheng, Yu.-Q., Zhang, J., and Liu, J.-Yo., CrystEngComm, 2010, vol. 12, p. 2740.
Chen, Yu-B., Zhang, J., Cheng, J.-K., et al., Inorg. Chem. Commun., 2004, vol. 7, p. 1139.
Ou, Y.-C., Liu, W.-T., Li, J.-Y., et al., Chem. Commun., 2011, vol. 47, p. 9384.
Tang, En., Dai, Yu-M., and Lin, Sh., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2004, vol. 60, p. m433.
Li, X.-M., Wang, Q.-W., Cui, Y.-Ch., et al., J. Huaxue (Chin.) (Chin. J. Struct. Chem.), 2006, vol. 25, p. 621.
Wu, Li-li., Rong, He., Zhen, W., Hui-Hua, S, Chem. Res. Chin. Univ., 2011, vol. 27, p. 724.
Wang, X., Qin, Ch., Wang, E., and Xu, L., J. Mol. Struct., 2005, vol. 737, p. 49.
Suresh, E., Boopalan, K., Jasra, R.V., and Bhadbhade, M.M., Inorg. Chem., 2001, vol. 40, p. 4078.
Tobin, J.M. and Mark, A.R., Angew. Chem., Int. Ed. Engl., 1995, vol. 341, p. 155.
Paul, A.M., Charlotte, L.S., and Kenneth, R.P., J. Am. Chem. Soc., 2001, p. 7742.
Robl, C., Z. Anorg. Allg. Chem., 1988, vol. 566, p. 144.
Vitiu, A.A., Coropceanu, Ed.B., and Bourosh, P.N., Russ. J. Coord. Chem., 2017, vol. 43, no. 11, p. 745. https://doi.org/10.1134/S1070328417110100
CrysAlis RED. O.D.L. Version 1.171.34.76, 2003.
Sheldrick, G., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, vol. 64, no. 1, p. 112.
Porai-Koshits, M.A. Zh. Strukt. Khim., 1980, vol. 21, no. 3, p. 146.
Baca, S.G., Simonov, Yu.A., Gerbeleu, N.V., et al., Polyhedron, 2001, vol. 20, p. 831.
Reger, D.L., Pascui, A.E., Smith, M.D., et al., Inorg. Chem., 2012, vol. 51, p. 11820.
Melnic, E., Coropceanu, E.B., Forni, A., et al., Cryst. Growth Des., 2016, vol. 16, p. 6275.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Vitiu, A.A., Coropceanu, E.B. & Bourosh, P.N. New Zn(II) and Cd(II) Coordination Polymers with 1,2-Benzenedicarboxylic Acid: Synthesis and Structures. Russ J Coord Chem 45, 81–91 (2019). https://doi.org/10.1134/S1070328419020106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328419020106